The term “Quality Metrics” means all those indicators that give an idea of the collective quality characteristics of a certain production site. The number of rejected batches per year compared to the total number of batches produced, or the number of out-of-specification (OOS) results found by a QC lab, and which are then not confirmed with respect to the total number of batches tested, are examples of generic indicators already used by most companies.
Quality Metrics Preparation Guideline
1.0 PURPOSE:
-
- The objective of this Standard Operating Procedure (SOP) is to provide the framework for quality metrics, and the reporting of key quality performance indicators related to product quality, process reliability, and overall effectiveness of the Quality Management System. Monitoring of quality metrics can result in continuous improvement initiatives and opportunities for technical innovation.
2.0 SCOPE:
-
- This SOP applies to the Quality Unit, Manufacturing, and support activities involved in the reporting and communication of quality performance indicating metrics at manufacturing sites.
3.0 REFERENCES:
-
- FDA Guideline – Measuring Quality Drives Excellence
4.0 RESPONSIBILITY – QUALITY METRICS:
-
-
Site management shall be responsible for –
-
-
- Demonstrating a commitment to the use of metrics to assess the state of quality at the site.
-
- Assuring appropriate metrics are selected to measure the quality attributes will drive success in meeting quality requirements for products, processes, and patients’ needs.
-
-
Regional quality head/designee shall be responsible for –
-
-
- Assuring all the sites operate in compliance with this standard.
-
- Communicating the authority of the Quality Unit within the organization to establish and manage quality performance indicating metrics.
-
-
Site quality head/designee shall be responsible for –
-
-
- Providing leadership required to site functional groups to assure alignment with this SOP.
-
- A leading site to establish written procedures to meet the requirements of this standard.
-
- Providing resources required to support data collection and assessments related to site metrics.
-
- Coordinating the collection of site quality performance indicating metrics and tabulating associated data into prescribed formats as required.
-
-
Site manufacturing, engineering/maintenance, planning, purchase, human resources (HR) shall be responsible for –
-
-
- Contributing to the collection of the site quality performance indicating metrics in all areas of operations and operational support as required.
-
-
Corporate quality compliance (CQC) head/designee shall be responsible for –
-
-
- Designing the Master Quality Metrics templates and communicating the requirements to the sites.
-
- Providing guidance and training to sites on the Quality Metrics.
-
- Reviewing the quality metrics received from sites, compiling data, and preparing the Quality and Compliance monthly report to be shared with senior management.
-
- Updating the quality performance indicating metrics as needed based upon signals, evaluation, or as requested by regulatory agencies.
-
- Establishing the frequency to monitor ongoing metrics and communicating to all manufacturing sites.
-
- Evaluating the Quality Metrics Monthly report.
5.0 ABBREVIATIONS & DEFINITION:
-
- API: Active Pharmaceutical Ingredient
-
- CCR: Change Control Record
-
- CPP: Critical Process Parameter
-
- FAR: Field Alert Report
-
- OOC: Out of Calibration
-
- OOS: Out-of-Specification
-
- PQR: Product Quality Review
-
- DEFINITION:
-
-
Active Pharmaceutical Ingredient:
-
-
- Active Pharmaceutical Ingredient is an ingredient intended to furnish pharmacologic activity or another direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the body; it does not include intermediates used in the synthesis of such an ingredient.
-
-
Annual Product Review/ Product Quality Review:
-
-
- An annual assessment as required by the EU was completed for each drug product and starting material produced at a given site compiling a broad range of Quality product/process indicators for products distributed in the EU.
-
-
Corrective and Preventive Action:
-
-
- A concept with current Good Manufacturing Practice (cGMP) that focuses on the systematic investigation of root causes of unexpected incidences to prevent their recurrence (corrective action) or to prevent their occurrence (preventive action).
-
- Corrective Action: Action taken to eliminate the causes of an existing nonconformity, defect, or other undesirable situation, in order to prevent a recurrence.
-
- Preventative Action: Action taken to eliminate the cause of a potential nonconformity, defect, or other undesirable situation, in order to prevent occurrence.
-
-
cGxP:
-
-
- cGxP is a general term that stands for current Good “x” Practice (x = Clinical, Engineering, Laboratory, Manufacturing, Documentation, Pharmaceutical, etc.). The titles of these Good “x” Practice guidelines usually begin with “Good” and end in “Practice”.
-
- cGxP represents the abbreviations of these titles where “x” a common symbol for a variable, represents the specific descriptor.
-
-
Change Control:
-
-
- Change control is a well-known cGMP concept that focuses on managing change to prevent unintended consequences. The cGMP regulations provide for change control primarily through the assigned responsibilities of the quality control unit. Certain major manufacturing changes (e.g., changes that alter specifications, a critical product attribute, or bioavailability) require regulatory filings and prior regulatory approval (21 CFR 314.70, 514.8, and 601.12).
-
-
Critical Process Parameter:
-
-
- A process parameter whose variability has an impact on a critical quality attribute and should be monitored or controlled to ensure that the process produces the desired quality.
-
- CQA Critical Quality Attribute: Chemical, physical, biological and microbiological attributes that can be defined, measured, and continually monitored to ensure final product outputs remain within acceptable quality limits.
-
-
Field Alert Report:
-
-
- Field Alert Report is a report submitted to the FDA outlined in the Code of Federal Regulation 314, Subpart 314.81, documented on Form 3331. Metrics is an assessment tool using figures or statistics to evaluate performance versus expected outcomes.
-
-
Out of Calibration:
-
-
- Out of Calibration (OOC), Status of GMP-related process equipment which has a past due date for calibration.
-
-
Out-of-Specification:
-
-
- All results that fall outside the pre-established specifications or acceptance criteria established in the drug application, drug master files, and official compendia or by the manufacturer.
-
- An OOS shall be investigated, documented, reviewed, and approved by the Quality Unit.
-
-
OOT Out-of-Trend results:
-
-
- Out of Trend (OOT), A result that is within the predefined limit (Specification limit), but is found to be not fitting the normal distribution of results, once an appropriate amount of data has been generated
-
-
Quality Risk Management:
-
-
- Risk Management is a systematic process for the assessment, control, communication, and review of risks to the quality of drug products across the product lifecycle.
-
-
Quality Review Board or QRB (also known as Quality Council):
-
-
- A forum to support the premise that the Quality Council is a mechanism to exercise management responsibility, as well as to ensure timely decisions and cross-functional support.
-
- It is a forum for leadership engagement, awareness, and decision-making around quality systems and process/ product performance.
-
- It is not a substitute for line management accountability or the only forum for addressing improvements to quality systems and cGMP problem-solving.
-
-
Quality Unit:
-
-
- Quality Department, including both Quality Assurance and Quality Control, functions. The Quality Unit has a broad range of authority at each manufacturing, packaging, or contract manufacturing site.
-
- This authority is dictated by GMP regulatory guidance and internal Policy and gives the Quality Unit the responsibilities to approve, reject, or quarantine GMP related material, to review cGxP documentation and approve if satisfactory, to test material for acceptability, to provide communication to regulatory agencies, to manage regulatory inspections, to manage cGxP documentation systems, to manage product recalls, to manage quality aspects of contract manufacturing, to provide oversight of cGxP training to manage the storage of product stability and retention samples and to assure correct expiry dating for the finished product.
-
-
Rapid Alert:
-
-
- Rapid Alert Reporting is required by regulatory agencies (e.g., European Medicines Agency and India Central Drugs Standard Control Organization) to transmit those alerts where urgency and seriousness of the event cannot permit delay in transmission.
-
- Reprocessing:
-
- Introducing material or product, including one that does not conform to standards or specification, back into the approved process and repeating one or more steps that are part of the established manufacturing process to obtain an acceptable quality product. Reprocessing is taking a material (in-specification or out of- specification) and reintroducing it to an existing (validated)
-
- Rework: Subjecting an intermediate or an API that does not conform to standards or specifications to one or more processing steps that are different from the established manufacturing process to obtain an acceptable quality product.
-
- Reworking is taking an out-of-specification product and running it through a non-standard process to bring it back into specification. Require Concurrent validation. For example, re-filtering a bulk liquid following an in-process.
-
- Right First Time: Completing a specific operation in the first attempt without incurring errors.
-
- Validation (Facility, Equipment, Utility):
-
- The activity of establishing documented evidence, which provides a high degree of assurance that a specific method, process, or system will consistently produce a result meeting its predetermined specifications and quality attributes
6.0 PROCEDURE – QUALITY METRICS:
-
-
Site Metrics Applications:
-
-
- Ongoing Metrics:
-
- Statistical assessments of CQAs, CPPs, yields, and laboratory analytical test results are part of Continued Process Verification.
-
- Sites shall provide Ongoing metrics data as per frequency defined by CQC.
-
-
The following metrics as applicable shall be monitored for formulation and API areas, but not limited to:
-
-
- Batch rejection rate / API Lot failure rate.
-
- Events/incidents (total number initiated and overdue for closure).
-
- Repeated deviations/incidents (number of occurrences and percent of total events).
-
- FARs (total number overdue as a percent of total raised). vaccination
-
- Total recalls performed /Open Recalls.
-
- Percent recalls completed.
-
- The total number of reworked batches.
-
- The total number of reprocessed batches.
-
- Right first-time percentage.
-
- Change control (total number open beyond target completion date).
-
- The number of data integrity Good Documentation Practices (GDP) violations observed (refer to current version of SOP – Data Integrity Practices and the current version of SOP – Good Documentation Practices (GDP)).
-
- Product quality complaints (total complaints received, total complaint investigations overdue).
-
- Critical complaints (percent review and response completed on time).
-
- Attempted lots pending for disposition (On-hold batch rate).
-
- CAPAs (number initiated, number overdue).
-
- OOSs (total number, percent of total confirmed, total number overdue, invalidated OOS).
-
- OOTs (total number raised, percent of total number confirmed and total number overdue).
-
- Laboratory investigations (total number raised, total number open beyond target date).
-
- Stability (number of confirmed failures, number tested in overdue period, number overdue pending test).
-
- Plant up-keeping rating according to local SOP.
-
- Inspection/Audit (number of regulatory observations not addressed according to commitment, number of internal audit observations remaining open, number of open external audits).
-
-
The following metrics as applicable shall be monitored for the QA Operational Support area, but not limited to:
-
-
- Percent Regulatory audit responses sent on time.
-
- Percent Annual Product Review/Product Quality (APR/PQR) Reviews completed on time and if any are currently overdue.
-
- SOPs (percent of SOPs revised on time according to schedule.
-
- Document review (batch production documentation error rate).
-
- Preventive maintenance work orders (total number and percent completed on time).
-
- Calibrations (total number and percent calibrations completed on time).
-
- The number of validation activities overdue versus the total number of validation activities scheduled.
-
- Missed document reviews versus the total number of documents reviewed.
-
- Critical deviations/incidents by suppliers.
-
- Critical deviations/incidents by third-party manufacturers.
-
- Qualifications (percent scheduled qualification/ requalification performance verification completed on time).
-
- Vendor Qualification (percent of vendors/manufacturers not having complete files for qualification documents, audit reports, etc.)
-
- Line clearance (number of failed line clearances in packaging and manufacturing based on QA second check).
-
- Daily QA line observations (total number of critical/major issues, number of observations versus total number of audits).
-
- Scale-up (number of the scale-up reports pending as a percent of total scale-up lots manufactured).
-
-
The following metrics as applicable shall be monitored for QC Operational area, but not limited to:
-
-
- Up keeping (number of observations).
-
- Out of Calibration (number of calibrations initiated versus the number of calibrations overdue).
-
- Release time variation (percent meeting target release time).
-
- Review observations (number of documentation errors).
-
- Method transfer (percent of method transfer certificates in place for all products tested by company and contract laboratories).
-
- Instrument utilization (percent instrument availability used).
-
- Analytical issues (number of issues pending as a percent of the total number of issues reported).
-
- Any issues related to the stability study program.
-
- Calibration and maintenance (percent of scheduled calibration and maintenance activities completed on time).
-
- Instrument qualifications (percent scheduled qualification/requalification, performance verification completed on time) From all relevant areas, as applicable:
-
- Training completed on time and any outstanding training to complete.
-
- Analysis of data spanning several months/ quarters to assess trends for quality impact.
-
- Follow-up action items.
-
Requirements – Quality Metrics:
-
- Quality Review Board (QRB):
-
- Established metrics are reviewed at regularly scheduled QRB meetings to support an ongoing quality assessment of site key quality parameters.
-
- Establish quality expectations for levels of performance from a standardized metrics review at the regularly scheduled QRB meetings. Base quality expectations on Company quality initiatives, Regulatory expectations, and site requirements.
-
- Evaluate metrics and prioritize actions as required to address issues that negatively impact the quality results expected by internal or external requirements.
-
- Communicate results of metrics review and related expectations of the QRB to assure actions are in place via the Corrective Action/Preventive Action (CAPA) system to deliver Continuous Quality Improvement.
-
-
Quality Unit:
-
-
- Align necessary resources to manage and complete QRB directed assignments.
-
- Inform the QRB of any directed assignment resource allocation issues or other obstructions that may prevent the completion of assigned tasks within the agreed time period.
-
- For cross-functional QRB assignments, assure alignment and project understanding with the Manufacturing Unit and support areas to assure clarity of assignment, clarity of responsibilities and that agreement exists for specific resource allocations.
-
- Provide QRB project assignment updates at the regularly scheduled Quality Council meetings.
-
-
Manufacturing Unit and Support Groups:
-
-
- Support QRB project assignments as required.
7.0 ANNEXURES – QUALITY METRICS:
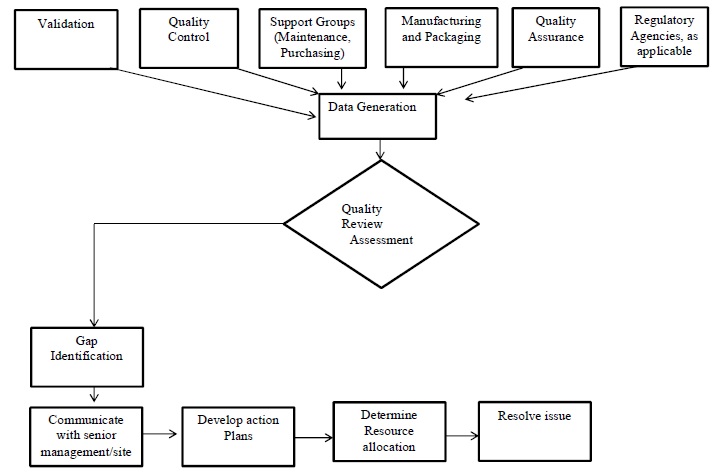
Annexure 1: Quality Metrics-Workflow.
***************************************************END***************************************************


