The drug product recall is a process of removal or correction of marketed products for the reasons relating to deficiencies in quality, safety or efficacy, including labeling considered to be in violation of the laws.
SOP for Product Recall

1.0 PURPOSE
-
-
The purpose of this SOP is
- Effective and speedy withdrawal/ removal of drug products from Market/ distribution during its shelf life period.
-
-
- Withdrawal/ removal of drug product from Market/ distribution for corrective action for deficiencies that are impacting the Strength, Identity, Safety, Purity, and Quality (SISPQ) of the drug product.
2.0 SCOPE
-
- This SOP shall be implemented as such for marketed Drug product recall, mock recall at the pharmaceutical manufacturing plant.
3.0 RESPONSIBILITY
-
-
Quality Assurance Department (QA) shall be responsible for :
-
-
- Investigation for product failure after release.
-
- Initiation of Product Recall.
-
- Initiation of Mock Recall.
-
- Maintaining the Documents and LOG for recall and Mock recall.
Also, read => SOP for Change Control Management
-
-
Head Quality Assurance shall be responsible for :
-
-
- Investigation for product failure after release.
-
- Review & Approval of product/Mock recall.
-
- Reporting Investigation findings to Management.
-
- Taking necessary actions to ensure speedy, effective and efficient product recall and Mock recall.
-
- Intimation to Management, Marketing Head, Distribution Head and Warehouse Head for recall and Mock recall.
-
- The decision for impacted/recalled quantity.
-
- Intimation to Regulatory authorities.
-
-
Head Corporate Quality shall be responsible for :
-
-
- Review and Approval of product recall.
-
- Intimation to Regulatory Authorities.
-
-
Management shall be responsible for :
-
-
- Authorization for product recall.
-
- Providing necessary support to ensure speedy, effective and efficient recall and Mock recall.
-
-
Distribution Head shall be responsible for :
-
-
- Maintaining the records of distribution.
-
- Informing branch managers about the product recall.
-
- Taking necessary actions to ensure speedy, effective and efficient recall and Mock recall.
-
- Support and Coordination for recall and Mock recall.
Also read => SOP for Vendor Management
-
-
Marketing Head shall be responsible for :
-
-
- Stop marketing of recalled / Mock Recalled product/batch.
-
- Taking necessary actions to ensure speedy, effective and efficient product recall and Mock recall.
-
- Support and Coordination for recall and Mock recall.
-
-
Production Head shall be responsible for :
-
-
- Coordination and support in the Investigation.
-
- Checking of Batch records etc.
-
- Taking necessary corrective actions and preventive actions (If required).
-
-
Warehouse Head shall be responsible for :
-
-
- Providing distribution details of related batch/product.
-
- Reconciliation and proper handling/storage of recalled products.
-
- Destruction of the recalled product.
-
-
Plant Head shall be responsible for :
-
-
- Review and approval of product recall.
4.0 PROCEDURE FOR PRODUCT RECALL :
-
Investigation Prior to Product Recall :
- Any written communication received from a complainant (Distributor / Retailer / Stockiest / Field Staff / Customer / Doctor) regarding the defects in the product quality (purity, efficacy or any adverse drug reaction) including its physical characteristic(s), packaging, labeling, etc. shall be investigated as per the “SOP on Handling of Market Complaints”.
-
- Based on the recommendations given in the Market Complaint Investigation report, Head – Quality Assurance shall initiate the recall of the product if required.
-
- In case, it is found that other batches of the same product or even of other product(s) have the same defect, the time frame for investigation may increase.
Also, Read => Record Retention and Archival Policy
-
Reason for Product Recall :
- Head – QA (Site) shall initiate the product recall in the following circumstances but not limited to:
-
-
- Official notification received from Drug Regulatory Authorities.
-
-
-
- Recall initiated by the company as a result of abnormal observation in any product quality.
-
-
Classification of defects :
- Based on the severity of defects and their adverse health consequences “DEFECTS” are classified as:
-
Class I Defects:
-
- Class I defects are potentially life-threatening or situations in which there is a probability that the use of a product will cause serious adverse irreversible health consequences or death.
-
- In such cases, A Rapid Alert notification must be sent to all contacts of the rapid alert notification list, irrespective of whether or not the batch was exported to that country.
-
- Recall of this class may extend up to the consumer level.
-
- Examples of Class I defects (but not limited to):
-
-
- Regulatory bodies issued a withdrawal notification in class I.
-
-
-
- Wrong product (Label and content are of different products).
-
-
-
- Correct product but wrong label strength, with serious medical consequences.
-
-
-
- Chemical contamination with serious medical consequences.
-
-
-
- Microbial contamination in sterile injectable
-
-
-
- Mix-up of products (rogues).
-
-
-
- Wrong active ingredients in a multi-component product, with serious medical consequences.
-
-
-
- All banned drugs & products for which license is suspended, canceled which are in circulation.
-
Also read: SOP for Document Management System
-
Class II Defects:
- The situation in which the use of, or exposure to a defective product may cause illness or mistreatment or may cause temporary or medically reversible adverse health consequences is remote but are not Class I.
-
- A Rapid Alert notification should be sent to all contacts of the rapid alert notification list as it might be difficult to know where a batch has been distributed.
-
- If the product distribution is known, the notification should be only sent to the contacts concerned.
-
- Examples of class II defects (but not limited to):
-
-
- Mislabeling, eg. Wrong or missing text or figures.
-
-
-
- Non-compliance to specification (e.g. dissolution, disintegration, assay, fill weight, stability).
-
-
-
- Microbial contamination in non-sterile products with medical consequences.
-
-
-
- Missing or incorrect information (Leaflet or inserts), any labeling/leaflet misinformation (or lack of Information) which represents a significant hazard to the patient.
-
-
-
- Chemical/Physical contamination (Significant impurities, cross contamination, Particulate or Extraneous matter).
-
-
-
- Leakage of content or sealing defects.
-
-
-
- Mix-up of products in containers (rogues).
-
-
-
- Physical or visual deterioration like significant discoloration, sedimentation, and crystallization.
-
-
-
- Insecure closure with serious medical consequences (eg: Child-resistant container).
-
-
Class III Defects:
- Class III defects may not pose a significant hazard to health, but withdrawal may be initiated for other reasons.
-
- These are not normally notified through the Rapid Alert System.
-
- If deemed relevant by the issuing authority, the rapid alert system may be used.
-
- Examples of Class III (but not limited to):
-
-
- Faulty packing e.g. wrong or missing batch number or expiry date.
-
-
-
- Any physical defect e.g. chipped tablet, broken tablet, cross label, shortage, etc.
-
-
-
- Faulty closure
-
-
-
- Contamination, eg. Microbial spoilage, dirt or detritus, particulate matter.
-
Also read: Process Validation SOP and Protocol
-
Product recall type :
- Recall of product shall be classified into the following three types:
-
- Statuary recall.
-
- Voluntary recall.
-
- Mock recall.
-
Statuary Recall of Product:
- A recall directed by Drug control authority / Regulator or any other equivalent authority, after notifying that the product is considered to be a violation of laws e.g:
-
-
- Declared as not of standard quality by Government analyst or qualified person in case of export.
-
-
-
- Banned by DCGI or Drug authority / regulatory agency of that country.
-
-
Voluntary Recall of Product:
- A recall initiated by the company as a result of abnormal observation in any product quality;
-
- Trigger the Voluntary recall by an incident that affects the Strength, Identity, Safety, Purity, and Quality (SISPQ) of drug product/batch in question such as;
-
-
-
- During the periodic review of the product.
-
-
-
-
-
- The batch or batches found not complying with the regulatory specifications during the post-marketing stability study.
-
-
-
-
-
- If the batch is found defective during the investigation of the market complaint.
-
-
-
-
-
- During any failure investigation, if it is observed that the failure under investigation might have an adverse quality impact on the already released batch (e.g. possibility of contamination, mix-up, degradation, etc.).
-
-
-
-
-
- If any unusual observation is noted during a visual inspection of retention sample which indicates an impact on the quality of the product after investigation.
- It the post-marketing surveillance reports/pharmacovigilance reports indicate that there is serious safety risk associated with the product.
-
-
-
Mock Recall :
- A recall carried out to check the effectiveness of the recall.
-
- Mock recall shall be carried out at least for one product, dispatched for sale where maximum distributors are involved and details shall be recorded in Annexure 5 – Product Mock Recall.
-
- Mock recall shall be performed for the longest distribution chain at least once in two years.
-
- During mock recall, Perform the traceability for at least, one of the raw materials used in the batches identified for mock recall.
Also read: Technology Transfer of Drug Product
-
- The depth of mock recall shall be up to the stockiest level only.
-
- Mock recall shall be aimed at the following objective:
-
-
- Alert recall team
-
-
-
- Performing the reconciliation to test product traceability.
-
-
-
- Test the responsiveness and knowledge of the team operating the recall system
-
-
-
- To implement any corrective action to improve the recall system.
-
-
-
- Mock Recall process shall be completed within 30 working days from the date of initiation.
-
-
Product Recall Co-ordination committee :
-
-
- The details of the Recall Co-ordination Committee shall be prepared as per section C of Annexure 1 – Product Recall Initiation Form.
-
-
-
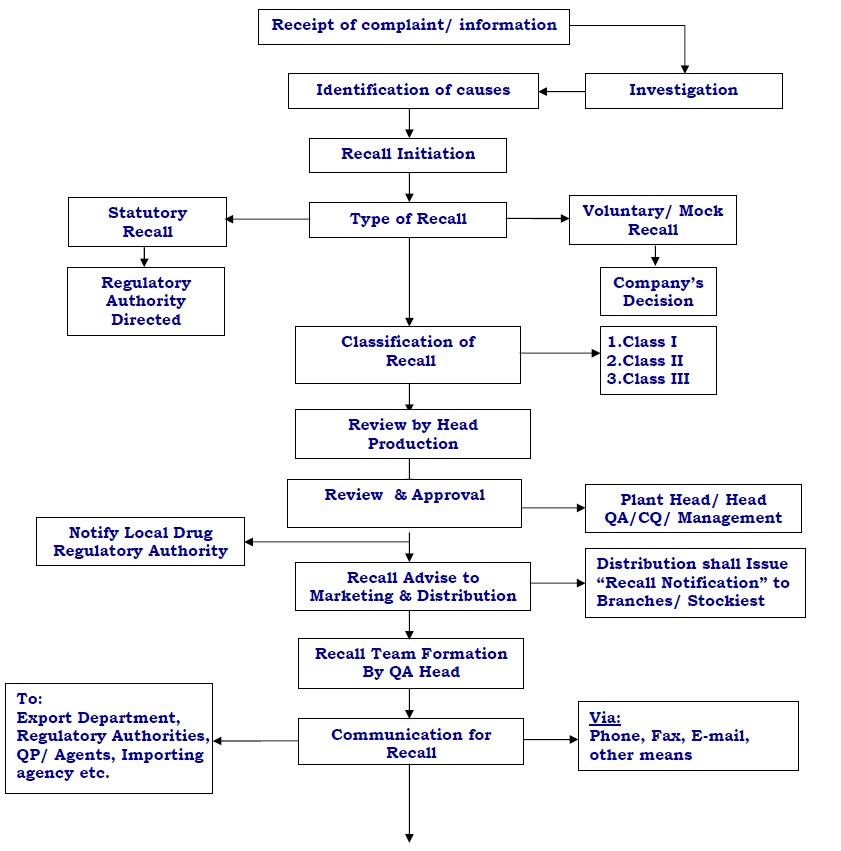
Initiation of Product recall :
- Based on the investigation finding & reports, once decided that the product needs to be recalled, the Technical staff of QA shall initiate the recall as per the Annexure 1 – Product Recall Initiation Form and
-
- LOG shall be maintained as per Annexure 4 – Logbook for Product/Mock Recall.
-
- The technical staff of QA shall allot the recall initiation No. as per below procedure:
-
-
- Each product recall shall be allocated a recall no, comprises 12 (Twelve) alphanumeric characters as:
- XX/AA/YY/ZZZ
- Where;
- XX Stands for location code (manufacturing plant)
- / : Slash
- AA: Stands for (“PR” Product Recall and “MR” for Mock Recall)
- / : Slash
- YY: Last two digit of the current year
- / : Slash
- ZZZ: Serial number i.e. 001, 002 ……….n.
-
-
-
Recall initiation form shall be sent to the Head- Production, and Plant-Head for review.
-
-
- After review from the plant head, the recall initiation form shall be approved Head-QA.
-
- Head-QA shall forward the approved recall initiation form to Head-CQ through electronic mail/post/courier for final approval.
Also visit: SOP for Control Sample Management
-
- After getting the approval from Head-CQ, Head-QA shall forward the same to Head Distribution and Marketing Head along with distribution details for initiation of Recall and parallel to management for their information /acknowledgment.
-
- Head-QA shall inform Manufacturing license FDA / respective regulatory authorities for the product recall.
-
- Head-QA/Head-CQ shall inform the competent authority of all countries to which product may have been distributed and to the importing agency (if applicable) through the export department.
-
- The warehouse head shall provide the distribution details of the product/batch to QA.
-
- The technical staff of QA shall check the distribution record to identify the warehouse to which the subject product/batch numbers have been sent.
-
-
Head QA shall send the product recall advice as per Annexure 2 – Product Recall Advice to distribution and marketing, along with distribution details.
-
-
- QA HOD shall inform to local FDA about the location where the product/batch has been distributed immediately after the decision for the recall has been taken.
-
- Head Distribution/designee shall plan the implementation of recall and start the process by ‘Recall Notification’ to Warehouses/wholesalers / Physicians / Pharmacies / retails outlets / Hospitals depending upon the level of the recall.
-
- The distribution head shall fill the “Recall Notification” as per Annexure 3 – Product Recall Notification and the same shall be sent to the above-mentioned places/ concerned persons to block for distribution and get the stock statement available at their end.
-
- The distribution head shall ask stockiest to notify the retailers and advise them to stop further sale of the product/batch for which recall has been initiated as per Annexure 3 – Product Recall Notification .
-
-
The remaining stock shall be sent back to the factory / central warehouse.
-
-
- Based on the distribution data, the Distribution Head or his designee communicates (telephonic communication is immediately confirmed in writing) the decision to the relevant key points in the distribution chain.
-
- Considering the severity of the cause of “Recall”, the first message to the relevant key point in the distribution chain shall be “STOP DISTRIBUTION / SALE & FREEZE STOCKS”.
-
- In case of Hospital/government supply, the distribution head/marketing head shall take up the matter with the concerned authority and ask them to block further use of product/batch and shall get the stock statement of total unused/used quantity.
-
- The distribution team shall ensure that all unsold stocks at all levels viz. stockiest, distributors, retailers are immediately quarantined and returned to warehouses/depots for further action.
-
-
The distribution head shall send a copy of such communication and acknowledgment of the same received from the branch stockiest and retailer to site QA head.
-
-
- Head Distribution/designee shall arrange to provide all the relevant distribution record/stock status to site-QA which shall contain sufficient information on wholesalers and directly supplied customers (with addresses, phone and/or fax numbers, inside and outside working hours, batches and amounts delivered), including those for exported products and medical samples.
-
- During recall activity of any product site, QA head shall be always in contact with Head – CQ & distribution head for all correspondence & development.
-
- Any communication to the regulatory authorities, government authorities, and institutions during the activity must be shared within the site QA head, distribution head & Head – CQ by telephone, fax or electronic mail.
-
- The recall process of collecting the stock under question and maintenance of proper and separate account of such stocks should complete within one (01) month of initiation of the “Recall”. This time limit may be compressed/ reduced to the best possible in “Life-threatening” situations.
-
Level of product recall and timelines :
- Recall Notification / Rapid Alert shall be initiated within 24 hours.
-
-
Class I:
-
-
-
- Initiate Product recall at the central warehouse, branches, stockiest, retailers, hospitals, pharmacists, dispensary and consumers.
-
-
-
- Marketing shall organize rapid alert to consumer-level through public announcements, prints / electronic media aids viz. newspapers, television, radio, etc.
-
-
-
- Marketing head shall send a specimen of all such communication and acknowledgment of the same received from the central warehouse, branches, stockiest, retailers, hospitals, pharmacists, dispensaries to site head QA.
-
-
-
- Initiate to process the product recall within 24 hrs.
-
-
-
Class II :
-
-
-
- Product recall shall be initiated at the central warehouse, branches, stockiest and retailers.
-
-
-
- Marketing head shall send a specimen of all such communication and acknowledgment of the same received from central warehouse, branches, stockiest and retailers to head QA.
-
-
-
- Head QA shall evaluate the requirement of inspection/samples for further investigation (as recommended in recall format).
-
-
-
- The product recall process shall be initiated within 24 to 72 hrs.
-
-
-
Class III :
-
-
-
- Product recall shall be initiated at the central warehouse, branches, stockiest.
-
-
-
- Marketing head shall send a specimen of all such communication and acknowledgment of the same received from the central warehouse, branches, stockiest to head QA.
-
-
-
- Head QA shall evaluate the requirement of inspection/samples for further investigation (as recommended in recall format).
-
-
-
- The product recall process shall be initiated within 10 days.
-
-
-
Follow-up action of Recalled Goods :
-
-
-
- Follow-up action consists of a check on the effectiveness of recall, an investigation of the reason for the recall and remedial action taken to prevent a recurrence of the defect.
-
-
-
- Head QA shall monitor the recall process to determine the recall is progressing satisfactorily.
-
-
-
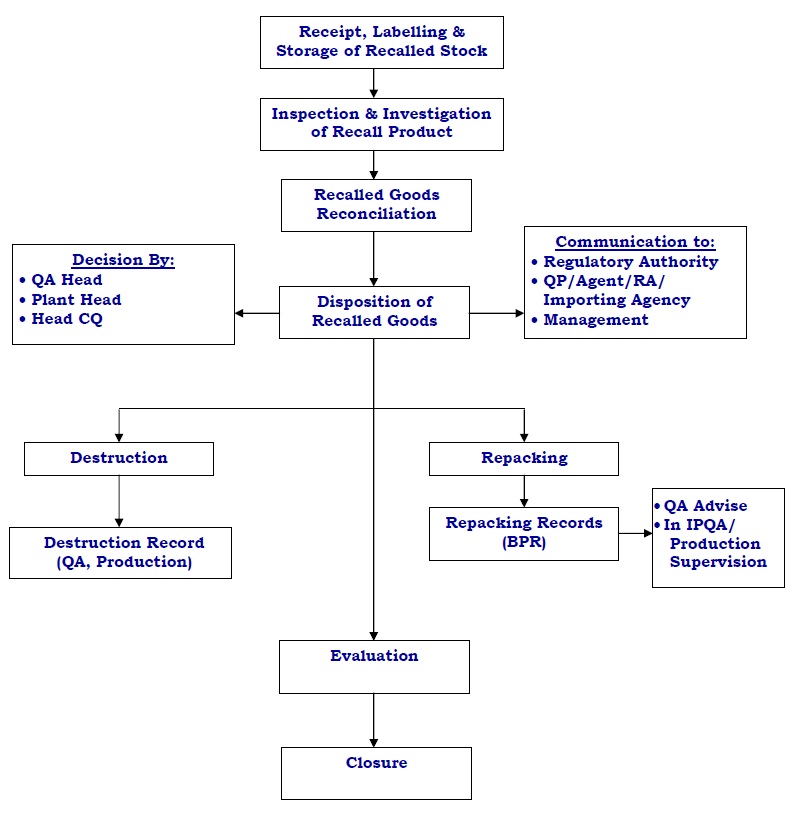
The holding of Recalled Product :
-
-
-
- Reconciliation of recalled goods shall be done and recorded in Annexure 1 – Product Recall Initiation Form in Section E.
-
-
-
- Recalled goods shall be placed under “Quarantine” and stored separately under lock and key in a secure area until further decision.
-
-
-
- QA shall perform a physical inspection of recalled goods and collect the sample from recalled goods for the investigation to establish the root cause of the product quality defect, where applicable.
-
-
-
Investigation of recalled product/batch :
-
-
-
- Investigation of the recalled products shall be conducted to identify the root cause of the failure and initiate corrective and preventive actions.
-
-
-
- The impact assessment shall be conducted on other batches of the concerned product and further extended to batch/s of other product(s), wherever applicable.
-
-
-
- In case the cause of recall is established to be a quality issue associated with raw material used, traceability of the material shall be established in all the product/batches it is used.
-
-
-
- Execute the transaction through records to identify the batches/ product in which the identified material has been used.
-
-
-
-
Review of relevant data i.e. Material, Plant (where raw material manufactured) and Batch Number in respective fields.
-
-
-
-
- List all raw materials along with analytical report numbers and respective quantities used in those batches.
-
-
-
- Calculate the total quantity by reconciling the total quantity of the RM used in various products/batches.
-
-
-
- Monitoring the material movement, to get a complete overview of stock for that particular material in plant and exact information about total quantity received and balance quantity.
-
-
-
- Balance stock, if any shall be verified against actual physical stock available. QA shall hold the remaining available stock.
-
-
-
- The decision to recall, if necessary, any of the impacted batches/product shall be made after product quality assessment.
-
-
-
Disposal of Recalled Product :
-
-
-
- QA shall inform & update FDA / Excise for recalled products, wherever applicable.
-
-
-
- After FDA / Excise clearance, QA shall arrange disposal of the batch/s, according to investigation findings, which may be repacking or destruction based on the reason for the product recall.
-
-
-
- Head QA shall decide the disposal of the recalled product in consultation with plant head and Head CQ within 30 days after receipt of the last consignment recalled product at plant warehouse.
-
-
-
- Perform the destruction as per respective SOP in the presence of QA representative and Security Person and document the same as per procedure and record the same in section F of Annexure 1 – Product Recall Initiation Form.
-
-
-
Evaluation of Product Recall :
-
-
-
- Check the effectiveness of every recall to verify that the recall notification letter was received by the customer/distributor, that the customer/distributor read & understood the letter and followed the recall instructions.
-
-
-
- Verify that recall has reached up to the appropriate (predefined) level in the distribution chain.
-
-
-
- If effectiveness checks indicate that the recall notification was not received, read and/or instructions were not followed, then necessary steps shall be taken to make recall effective.
-
-
-
- These steps may involve sending out a follow-up notification that better identifies the product/problems and or instructions.
-
-
-
-
Maintain a recall status report after initiating a recall.
-
-
-
-
- The report shall include the following information:
-
-
-
-
- Dates on which customers are notified
- Number of customers notified
- Number of a customer responding
- Quantity of recalled product returned or accounted for
-
-
-
-
- Establish the root cause of the problem to take appropriate corrective and preventive measures. which will prevent a recurrence of a similar problem.
-
-
-
- Enter the details of the evaluation in the Section-G of Annexure 1 – Product Recall Initiation Form.
-
-
Closure of Product Recall :
- Evaluate the product recall for closure after receiving all possible customer responses and the recalled product has been recovered/corrected/redressed/destroyed.
-
- Record of product recall shall be maintained by QA in Annexure 4 – Product Recall “Logbook for Product Recall”.
Flow Chart for Recall
5.0 REFERENCES TAKEN FOR PRODUCT RECALL PROCESS
-
- SOP for “Procedure for SOP design and management”
-
- SOP for “Procedure for Handling market complaint”
-
- PI010-5 Procedure for handling rapid alerts and recalls arising from quality defects (PICs guideline).
-
- Guideline on recall and rapid alert system for the drug (CDSCO/RRAS, 11/2012)
6.0 ABBREVIATIONS AND DEFINITION
-
- SOP: Standard Operating Procedure
-
- QA: Quality Assurance
-
- BPR: Batch Packing Record
-
- CQ: Corporate Quality
-
- QP: Qualified Personnel
-
- FDA: Food and Drug Administration
-
-
DCGI: Drug Controller General of India
-
-
-
Recall –
- Removal or correction of marketed products for the reasons relating to deficiencies in quality, safety or efficacy, including labeling considered to be in violation of the laws.
-
-
-
Batch Recall:
- Process for removal of selected batch/es of a product which are found to be defective and pose a health risk to the consumers if left in the market.
-
-
-
Batch (Lot):
- A specific quantity of material produced in a process or series of processes so that it is expected to be homogeneous within specified limits.
-
-
-
Customer:
- Any person, firm or party buying/receiving goods from the company for storage, distribution, and sale.
-
-
- Mock Recall: is an exercise, carried out by the manufacturer, in this case, there is no actual (voluntary or statutory) product recall. This exercise is carried out to challenge the effectiveness of the defined product recall procedure, over a due course of time.



Pingback: Cleaning Validation master plan (CVMP)-New Approach - Pharma Beginners
Pingback: SOP for Handling of Market Complaint - Pharma Beginners