Stability study sop prepared according to ICH guidelines with required stability study sample incubation, sample pullouts and analysis of samples and summary.
SOP for Stability Study of Drug Product
1.0 Purpose:

-
- The purpose of this SOP is to describe the procedure for sample collection, selection of batches, incubation, withdrawal, analysis, reporting, and evaluation, discontinuation, and documentation of stability studies of the drug products.
2.0 Scope of SOP for Stability Study:
-
- This procedure is applicable to carry out a stability study of the drug products manufactured at pharmaceuticals drug manufacturing location or drug formulation development location.
3.0 Reference, Attachments, and Annexures :
-
-
Reference:
-
-
- Asian Guideline on the stability study of the drug product.
-
-
Annexures:
-
-
- Stability Analysis Schedule and Cycle (Annexure – 1)
-
- Master Stability Schedule (Annexure – 2)
-
- Summary (Stability) Report (Annexure – 3)
-
- Stability Discontinuation Authorization Form (Annexure – 4)
-
- Stability Sample Labels (Annexure – 5)
-
- Monthly Schedule for Stability (Annexure – 6)
-
- Stability Sample Quantity Format (Annexure – 7)
-
- Placebo Preparation Record (Annexure – 8)
-
- Placebo Record Book (Annexure – 9)
-
- Stability Sample Reconciliation and Destruction Form. (Annexure – 10)
-
- Extension Form For Stability Sample Analysis (Annexure – 11)
3.0 Responsibilities as per SOP for Stability Study:
-
-
Analyst:
-
-
- Maintain the analysis schedule and other stability study-related documents.
-
- Store all the stability study results along with the associated documents with a sample test form, chromatogram, and other relevant documents.
-
- Analyze the stability study samples as per SOP
-
- Inform to Head QC or Designee in case of Out of trend and OOS results.
-
-
Stability Study Coordinator :
-
-
- Preparation of the stability study protocol.
-
- Receive stability Study Protocol, incubation and withdrawal of samples, samples analysis, reporting of the result, destruction, and discontinuation of stability study samples.
-
- To maintain the reconciliation of charged stability samples at every stability study station.
-
- Prepare the master and monthly stability study schedule as per the stability study protocol.
-
- Provide stability study reports, trend & evaluation to designated QA person for review/ submission.
-
- To issue the stability study template to the analyst as per the SOP.
-
- To prepare and update the stability study summary report and update all the documents related to stability.
-
- Evaluation of analytical data after analysis of the sample.
-
- Report any significant changes in accelerated conditions to the Head QC or Designee
-
- Prepare stability study summary reports, trends & evaluation.
-
-
Head QC or designee:
-
-
- Ensure for the receiving, scheduling, incubation and analysis of stability sample is performed as per the applicable Stability Study Protocol and SOP.
-
- Verify the stability sample schedule as per the Stability Study Protocol.
-
- Investigate in case of Out Of Specification result and Out Of Trend result.
-
- Review the Stability Study Summary Report, Discontinuation, Trend & Evaluation of stability study performed.
-
- Initiate an investigation in case of Out Of Specification (OOS) result/stability study failures.
-
- Establish an easy retrieval and secure archiving procedure of stability study documents.
-
- Inform any significant change and stability study failure results to QC Head or Designee along with Quality Head.
-
- To train the concern persons before the implementation of SOP.
-
-
Quality Assurance:
-
-
- Review the Stability Study Protocol.
-
- Issuance of Stability Study Protocol.
-
- Issue the stability study test request form to the Quality Control Department.
-
- Submit the stability study samples to the Quality Control.
-
- Prepare stability study samples using a similar container closure system as used for marketing the Drug Products.
-
- Review of master & monthly stability study schedule.
-
- To send the stability samples to QC for stability study and maintain the stability study sampling register.
-
- Review and implement stability-indicating methods.
-
- Evaluate and recommend the shelf life of Drug Products.
-
-
Quality Head, Regulatory Affairs and Plant Head:
-
-
- To authorize any stability study discontinuation
-
- To review and approve SOP.
4.0 Abbreviation and Definition of Terms used in SOP for Stability Study:
-
-
Abbreviation used in the SOP for Stability Study:
-
-
- API: Active Pharmaceutical Ingredient
-
- ACC: Accelerated Climatic Condition
-
- CQ: Corporate Quality
-
- FDD: Formulation Development Department
-
- LTC: Long Term Condition.
-
- LOD: Limit of Detection
-
- LOQ: Limit of Quantification
-
- M: Month
-
- MOC: Material of Construction
-
- NMT: Not More Than
-
- OOT: Out of Trend
-
- OOS: Out of Specification
-
- QA: Quality Assurance
-
- QC: Quality Control
-
- RRT: Relative Retention time
-
- SOP: Standard Operating Procedure
-
- TLC: Thin layer Chromatography
-
-
Definition of Terms :
-
-
-
Stability:
- The capacity of a drug product to comply with the specifications laid down for the duration of the shelf life assigned to it when stored under the conditioned stated on the label of the Containers/Packs.
-
-
- Evaluation Batch:
- Evaluation batches are the batches taken under planned deviation for some studies. e.g. recovery batches.
-
- Validation Lot: The lots/batches taken for validation study purposes.
-
- Routine Batch: Regular batches taken for annual stability study purposes.
-
- Date-In / Incubation Date:
- The date on which, received stability samples are incubated in the stability study chambers / Incubators and are base on the calculation of due date for the stability study station withdrawals.
-
-
Stability Study Stations:
- Stability stations are the due dates by which stability study samples are to be withdrawn from the stability chambers/incubators.
-
-
- These samples are kept in different temperatures and relative humidity condition chambers/incubators as per recommendations based on the ICH guidelines and or the specific Stability Study Protocols.
-
- Shelf life:
- The time period during which a drug product is expected to remain within the approved shelf-life specification provided that it is stored as per the conditions defined on the label.
-
-
Accelerated condition testing:
- Studies designed to increase the rate of chemical degradation or physical change of a drug product by using exaggerated storage conditions as part of formal stability studies.
-
-
- Long Term condition testing: Stability studies under the recommended storage condition for the proposed or approved shelf life for labeling.
-
-
Container closure system:
- The sum of packaging components that together contain and protect the dosage form, this includes primary packaging components and secondary packaging components, if the latter is intended to provide additional protection to the drug product. A packaging system is equivalent to a container closure system.
-
-
- Primary Packaging: Primary packaging is the packaging which is in direct contact with the drug product.
-
- Secondary Packaging:
- A secondary packaging component means a packaging component that is not and will not be in direct contact with the dosage form.
-
- Release specification:
- The combination of physical, chemical, biological, and microbiological tests and acceptance criteria to determine the suitability of a drug product at the time of its release.
-
- Stability Study specification:
- The combination of physical-chemical, biological, and microbiological tests and acceptance criteria to determine the suitability of a drug product throughout its shelf life.
-
- Photostability Study :
- Photostability is the capacity of a molecule product to remain intact and unaffected on light exposure and does not result in unacceptable change.
-
-
Significant Change during stability study:
-
-
- A 5% change in assay from its initial value or failure to meet the acceptance criteria.
-
- Any degradation product’s exceeding its acceptance criterion.
-
- Failure to meet the acceptance criteria for appearance, physical attributes, and functionality test (e.g., color, phase separation, responsibility, caking, hardness, dose delivery per actuation).
-
- However, some changes in physical attributes (e.g., softening of suppositories, melting of creams) shall not be considered as a significant change.
-
- Failure to meet the acceptance criterion for pH.
-
- Failure to meet the acceptance criteria for dissolution for 12 dosage units.
5.0 Procedure for Stability Study:
-
-
General Instructions for Stability Study:
-
-
- Incubate the amount of sample specified in the respective protocol including quantity for analysis as well as for investigation purpose in case of OOT/OOS results.
-
- Lable properly for all the samples incubated should be properly labeled with the condition, orientation and stability study intervals for traceability, and to facilitate the reconciliation while withdrawing a sample from the incubator.
-
- Prepare the stability study protocol as per SOP of “Stability Study Protocol, Template and Specification Preparation” Attachment- 1
-
- Withdraw the stability study sample and analyzed as per the stipulated time window/ due date defined in the protocol.
-
- Investigate any failure (OOS/OOT) observed during stability study and documented as per the investigation procedure specified in the SOP of OOS and OOT investigation respectively
-
- Whenever any batch is found failing in stability study after expiry interval, then there is no need to perform analysis of further intervals.
-
- For such products, Stability Study Protocol is to be revised and future stability study batches are to be monitored only up to expiry interval or up to the interval till the product is stable whichever is later.
-
-
The status of an on-going stability study should be periodically reviewed (at least once a year).
-
-
- The stability study followed, the number of batches incubated, the reason for monitoring, condition of stability incubator/walk-in chamber, any excursion or deviation of stability result trend, stability failure, etc. but not limited to, should be evaluated and appropriate action if required should be initiated.
-
- Conduct stability study testing on the dosage form packaged in the container closure system proposed for marketing (including, as appropriate, any secondary packaging and container label).
-
- Consider the first three batches for Long term and Accelerated stability study. (“First” means the product is manufactured for the first time at the location).
-
- Batches should be of the same formulation and packaged in the same container closure system as proposed for marketing.
-
- In the case of a new product, samples of three consecutive batches shall be kept for Accelerated and long-term stability studies.
-
- Three consecutive batches mean, three batches of each strength of product X manufactured, which can have batch numbers ABC0001, ABC0005 & ABC0007, where, in-between batch numbers can be of any other product.
-
- In case of specific requirements, QA shall provide the samples to QC for stability study analysis accordingly.
-
-
Storage condition of Stability Study Samples:
- Refer Table-1 for different temperature and humidity conditions for the long term, Accelerated and intermediate condition as follows.
-
Table-1 (Storage condition)
RecommendedStorageCondition |
Stability Sample Incubation Condition |
|||
Accelerated |
Long term |
|||
Temp. |
% RH |
Temp. |
% RH |
|
|
Cool Place / Room Temperature Product |
40°C ±2°C |
75% + 5% |
30°C + 2°Cor25°C + 2°C |
75% + 5% 60% + 5% |
-
-
Stability Specification :
-
-
- The testing requirements shall be defined in the Stability Study Protocol and shall cover as appropriate, the physical, chemical, microbiological preservative, and functionality tests.
-
- Stability acceptance criteria should be derived from the consideration of all available stability study information.
-
- It may be appropriate to have the justifiable difference between the Stability and release acceptance criteria based on the stability study evaluation and the changes observed on storage.
-
- In the case of the pharmacopoeia drug products, criteria of release and stability specification will be as per monograph.
-
- In such a case tighter internal release limit needs to be retained to get drug products within the criteria during the shelf life.
-
- Whenever specification is updated for any reason (like pharmacopoeial change but not limited to) and causes a change in analytical method, acceptance criteria, test inclusion/deletion, etc. update the associated Stability Study Protocol which shows the required changes e.g. specification No., acceptance criteria, etc.
-
- If, after the change of analytical method immediate analyzed stability study station samples fall under the criteria of OOT or OOS then investigation shall be performed using the previous analytical method before concluding that it’s a stability study impact or a method impact on the drug product.
-
-
Stability Study Protocol :
- QA shall submit a request with all relevant information required for protocol preparation for the quality control department.
-
-
- Stability Coordinator/designee of the quality control department shall prepare the Stability Protocol (based on requirement and project).
-
- Prepare the stability protocol for the different requirements e.g. different packs, customer and regulatory agency, etc.
-
- QA shall review and approve the protocol.
-
- Issue the stability study protocol prior to the execution of the stability study.
-
- The sample quantity charged per station shall be 1.5 times of the actual required quantity for one-time analysis /per batch /per station/ storage condition. If not then it should be justified.
-
- Additional sample quantity shall be incubated/date in for accelerated & intermediate stability study conditions, equivalent to two stability study station quantities for chemical analysis & one stability station quantity for microbiology analysis.
-
- For Long Term condition, three stability station sample quantity for chemical analysis and one stability station sample quantity for microbiological analysis should be kept to perform any analysis OOT, OOS, Regulatory deficiency analysis, and retesting because of analytical method revision, etc.
-
- The change control procedure shall be followed if the protocol to be revised based on the requirement.
-
- QA shall refer to or confirm the stability sample quantity from the stability study protocol.
-
-
Initiation of stability :
-
-
- The quality assurance shall select the batches for stability studies.
| Category | Stability storage condition | Selections of batches |
| New drug product | Long term, ACC & Intermediate (if applicable) | First Three batches |
| Annual addition batch | Long term | One batch |
| Changes in the primary packaging/closure material/system (lining, rubber, type of sealing, cap and sterilization process) | Long term & ACC | One batch
Note: Three batches shall be charged if the change in the MOC of primary packaging (evaluation of selecting the No. of batches shall depend upon the type of changes) |
| API source change | Long term ACC | One batch |
| Simulated bulk transfer pack | Long term | As per respective protocol |
Change in component & composition |
||
| Those changes are unlikely to have a detectable impact on the formulation quality & performance | Long term | One |
| Those changes could have a significant impact on the formulation quality and performance | Long term & Accelerated | One |
| Changes that are likely to have a significant impact on the formulation quality and performance | Long term & Accelerated | Three |
Changes in the manufacturing site |
||
| Changes within the same facility | _ | No batch |
| Changes within a contiguous campus or adjacent blocks. | Long Term & Accelerated | One |
| Change to a different manufacturing campus | Long Term & Accelerated | Three |
Change in Batch size |
||
| Change in batch size up to including the factor of 10 times the batch sizes of the pilot batch. | Long term | One |
| Change in batch size up beyond factor of 10 times the batch sizes of the pilot batch. | Long term & Accelerated. | One |
Changes in manufacturing Equipment |
||
| Changes from non-automated to automated equipment or alternatives equipment of the same design and operating principles of any capacity | Long Term | One |
| Changes in equipment to a different design and different operating principle | Accelerated & Long term. If a significant body of information available. | One |
| Accelerated & Long term if the significant body of Information not available. | Three batches | |
Changes in the specified manufacturing Process |
||
| Changes involving adjustments to equipment operating conditions within the original approval range. | _ | None |
| Changes involving adjustments to equipment’s operating conditions outside the originally approved range. | Accelerated & long term | One |
| Change in the type of process | Accelerated & long term | Three |
-
-
Details of changes as per level 1, 2, 3 :
- *Level 1 change:
- Deletion or partial deletion of an ingredient intended to affect the color or flavor of formulation, or change in the ingredient of the printing ink to another approved ingredient.
-
-
- Changes in excipients expressed as a percentage (w/w) of the total formulation, less than or equal to the following percent ranges.
-
- The total additive effect of all excipients change shall not be more than 5% example if a product is consisting of active pharmaceutical ingredient, lactose, Microcrystalline cellulose, and magnesium stearate, the lactose, and microcrystalline cellulose shall not vary by more than an absolute total of 5%.
Table-I
| Excipient | % Excipient (w/w) out of total target dosage form weight |
| Filler | ±5 |
| Disintegrant, Starch | ±3 |
| Others | ±1 |
| Binder | ±0.5 |
| Lubricant Calcium Stearate/Mg Stearate | ±0.25 |
| Other | ±1 |
| Glidant Talc | ±1 |
| Others | ±0.1 |
| Film coat | ±1 |
-
-
*Level 2 changes:
- Change in the technical grade of an excipient (example: Avicel PH 102 changed with Avicel PH 200).
-
-
- Changes in excipient expressed as % w/w of total formulation greater than those listed above for a Level 1 change, out less than or equal to following percent ranges ( which represent a two-fold increase over Level 1 change refer Table-II.
Table-II
| Excipient | % Excipient (w/w) out of total target dosage form weight |
| Filler | ±10 |
| Disintegrant, Starch | ±6 |
| Others | ±2 |
| Binder | ±1 |
| Lubricant Calcium stearate/Ma stearate | ±0.5 |
| Other | ±2 |
| Glidant Talc | ±2 |
| Others | ±0.2 |
| Film coat | ±2 |
-
- The total additive effect of all excipients changes shall not vary by more than 10%.
-
-
*Level 3 changes
- Any qualitative and quantitative excipient changes beyond the ranges noted in level 1 and level 2.
-
-
-
Stability Study Test Request and sample receipt :
-
-
- Stability section head or designee shall intimate the QA in Annexure-7, regarding the stability study sample quantity required for stability studies.
-
- The number (Quantity) of Stability samples for drug products shall be withdrawn as per the Stability protocol prepared by the Stability coordinator or designee.
-
- QA shall withdraw stability samples randomly during on line packing after verifying in-process checks & FP results within the limit. It should be in the final pack so as to simulate the market pack.
-
- QA shall enter the details like S. No., Product Name, B. No., Type of stability , Sample Withdrawn on, Sample Withdrawn by & Remark/Reason for stability in QA stability sampling register.
-
- After sampling, QA shall send the sample to QC.
-
- Stability Coordinator or designee shall verify the stability study sample and its respective details like Stability condition, Received sample quantity, Sample received by/ date, etc. in the Stability Test Request Form as per respective SOP format.
-
- Samples shall be stored at their product storage conditions till the time it is not transferred to the stability chamber for incubation.
-
-
Stability studies shall be performed on each strength and pack size of the drug product.
-
-
- Based on Stability conditions and stations samples shall be reconciled, labeled and distributed in the respective stability chambers as defined in Stability Protocol.
-
- Samples for all stability conditions shall be incubated on the same date in all respective Stability chambers.
-
- Schedule the stability Program and make an entry in the Master Stability Schedule (Annexure-2).
-
- Make an entry in the Monthly Schedule for Stability (Annexure-6).
-
- Based on Stability conditions and stations samples shall be reconciled, labeled and distributed in the respective stability chambers as defined in Stability Protocol.
-
-
Labeling and Incubation of Stability Study Samples :
- Affix the colored label (Annexure-5) on each of the show boxes.
-
-
- Incubate the samples as per the required storage conditions mentioned in the intimation slip for the stability study.
-
- The temperature and humidity controller shall be calibrated as per schedule.
-
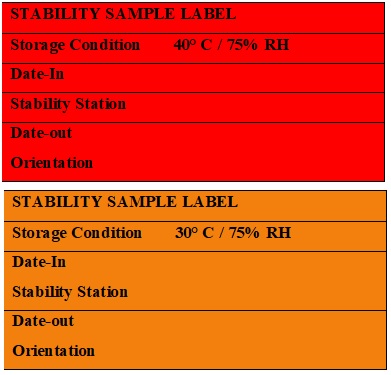
- The red color label shall be affixed on strips /show box of ACC condition and Orange color label shall be affixed on strips /show box of LTC condition as per Annexure -5.
-
- If the colored label is not available then colorless labels can be used.
-
- Samples which need specific orientation of pack shall be labeled with “Upright”, “Inverted” or “Horizontal”.
-
- Samples for all stability conditions shall be incubated on the same date in all respective Stability chambers.
-
- In the case of receiving specific requirements, Head QC or Designee can instruct to incubate the sample as per required stability study conditions.
-
-
Note :
- Stability study sample storage conditions are based on climatic zone III and IV, which can be changed as per the need for product registration in different countries and respective climatic zone.
-
-
- Example: Stability study conditions for some products of Vietnam are as below, Temperature: 30°C+2°C and Humidity: 75%+5%.
-
- The stability coordinator shall incubate the samples of the stability product to the respective chamber in the specified quantity and mark the samples for its identity.
-
-
List of Authorized persons with Stability study chamber access right shall be maintained.
-
-
- Entry to Stability Chamber shall be restricted, Lock and the key facility shall be available for stability chambers with limited access to chambers keys, documents and the usage of the same.
-
- Entry and Exit time shall be recorded with the sign and date of the person performing the activity.
-
- Preferably quality control analysts or personals involve in analysis shall not withdraw samples from the stability chamber. However, they may withdraw samples as and when required with the authorization of QC Head.
-
- After incubation of samples, analysis due date at required stations with ref. page no. of monthly stability schedule shall be mentioned in Annexure-6 & humidity of stability incubator shall be monitored on a daily basis.
-
- In case of any malfunctioning, inform the same to the engineering for its rectification.
-
- According to the stability schedule, the Stability coordinator shall schedule the analysis planning and issue the stability template for stability analysis.
-
- Any batch packed later or samples are provided after three months from the date of manufacturing then the samples should not be kept for stability studies.
-
- The stability coordinate shall draw the samples of the due product station from the respective chamber in a specified quantity at the time of analysis.
Also read => SOP for Vendor Management
-
-
After withdrawal, keep the sample in a suitable controlled temperature area or at 2-8°C if required.
-
-
- Mention the details on the label as Storage condition, Date-In, Stability Station, Date-Out, and Orientation (if applicable) (Annexure-5).
-
- Samples details, samples quantity date in/incubated like how many samples kept for chemical analysis, microbiological analysis, & samples kept for investigation shall be mentioned in reconciliation and destruction form.
-
- Whenever samples were withdrawn. Record the sample quantity in the reconciliation form.
-
- Submit the stability samples to quality control within 7 calendar days from the final packing date of the respective batch.
-
- Incubate stability samples in stability chambers at respective storage conditions specified in the Stability Protocol within 15 calendar days from the date of receipt.
-
- If the charging is delayed by more than 30 calendar days from the QC release, then the initial analysis shall be carried out again for the tests which are defined in either Stability Protocol or as required by Stability specification.
-
-
Consider results generated from this analysis as “0” Day results for the comparison of stability analysis across the stability study.
-
-
- If the finished product analysis method and stability-indicating method is the same, the initial finished product analysis result shall be considered as 0-month (Initial) for stability data.
-
- If finished product analysis method and stability-indicating method for specific tests are different, then “0” month (Initial) analysis of that particular test shall be carried out separately and other test results from finished product COA shall be considered.
Also, Read => Record Retention and Archival Policy
-
- Delay in incubation due to above or any other reason shall be addressed through event/incident this shall include proper justification and impact analysis.
-
- Samples received for the stability study. Store under control room temperature conditions before incubating to the stability chambers.
-
- Note: Products which require some specific temperature and relative humidity condition or highly sensitive to changing environmental condition shall be taken care as per the label claim or recommendation conditions and shall be incubated to stability chambers immediately.
-
- In case of batch incubated into stability study incubator after one month as based on date of manufacturing, in such case, additional station analysis shall be performed at actual shelf life and shelf life/expiry intervals are to be calculated from the date of manufacturing not from the date of incubation, and subsequent station as per the schedule also to be performed (based on market and regulatory affairs requirement).
-
- Any batch is manufactured in the last week of the previous year and packed in the new year in such a case batch should not be selected for the annual additional stability study.
-
-
Select Batch which is manufactured and packed in the same year.
-
-
- Keep at different places then the routine stability / regular release samples (to avoid mix-up) on control room temperature or as per the product storage conditions defined on the label.
-
- Use these samples only to respond to regulatory queries.
-
- For withdrawal of the stability samples, stability chambers shall be open single time or as and when required, remarks shall be given in case the stability chamber is opened more than once.
-
- In case, if the sample not withdrawn as per the schedule, justification has to be given for the same.
-
- Transfer the drawn samples to the Stability Sample Area in the Quality Control department.
-
- Stability In-charge shall make necessary entries in the Stability template issuance register and issue the template to analyst.
-
- Stability In-charge shall make entry of Sample withdrawal in Stability Sample reconciliation and destruction logbook for Qty. drawn, Qty. drawn date/by, Station No. / Due on, Qty. balance and Remarks as per (Annexure -10).
-
-
Handling and preparation of Placebo :
-
-
- Calculate the quantity of raw material and other API, if applicable, except concerned API in tablet per milligram from respective product BMR.
-
- Prepare the Placebo quantity as per requirements by mixing of raw material and record all the observation in Annexure-8.
-
- Store the Placebo of product at ambient temperature.
-
- Fill all the data in placebo preparation record like “Name of the product”, “Reference BMR No.”, “Placebo No.”, “Date of Preparation”, “Use before” and “Quantity Prepared”.
-
- Store the placebo bottle in Humidity control Oven/Chamber as per the product stability condition or designated place in the laboratory.
-
- Numbering System for Placebo
-
-
- PL/XXX
-
-
-
- PL stands for Placebo
-
-
-
- XXX stands for serial no. like 001,002,003…..etc.
-
-
- The analyst shall maintain the logbook for placebo traceability purposes as per Annexure-9.
-
- In case the placebo is not available, Consider reference chromatogram from previous analysis for Placebo peaks on the basis of its RRT.
-
- Incubate placebo along with the sample for individual stability study stations
-
- Assign the valid quantity of placebo (from date of preparation) for three years.
-
- Use the placebo up to product Long term study. (If any abnormal observations in the physical appearance of a placebo, discard it and prepare the fresh placebo).
Also, Read => Record Retention and Archival Policy
-
-
Scheduling Stability Study and Stability Testing frequency:
- Prepare the stability study master schedule based on stability protocol by QC & shall be verified by QA ( Annexure-2.)
-
-
- Prepare the monthly schedule for stability sample withdrawals, based on a master schedule (Annexure-2.)
-
- Plan the analysis for due stability stations or time points according to the monthly stability schedule which has been prepared by QC & verified by QA.
-
- After completion of analysis Stability Coordinator or designee shall make entries for “Analyzed by/date”, “Analysis reference document No.” in the monthly stability schedule as per Annexure-6.
-
- Stability Coordinator or designee shall fill the “Stability Sample Reconciliation and Destruction Form” and enter the following detail: “Product”, “B. No.”, “Quantity”, “Date-In”, “Condition” at the time of incubation as per Annexure-10.
-
- Stability Coordinator or designee shall make entries in the master stability schedule register as per Annexure-2. At the time of sample incubation, the Stability Coordinator shall make an entry for “Sample date-in”, “Sample Qty.”, “Stability station” & “Remarks” in the master stability schedule.
-
- Based on the “Master stability schedule” Stability Coordinator shall make an entry in the “Monthly stability schedule” register as per Annexure-6.
-
-
Note:
- Wherever LIMS application is a concern. Follow the procedure as per LIMS SOP.
-
-
- Where required (For data generation, submission requirement etc.) additional station or time point samples will be incubated at the same storage conditions and analyzed between the initial and final time point for the accelerated study, This must be defined in the protocol.
-
- For long-term studies, the frequency of testing shall be sufficient to establish the stability profile of the drug product.
-
- e.g. For products with a shelf life of 36 months, the frequency of testing at the long term stability shall be every 3 months over the first year, every 6 months over the second year, and annually thereafter through the shelf life (e.g. 0 Month, 3 Month, 6 Month, 9 Month, 12 Month, 18 Month, 24 Month, 36 Month).
-
- The additional intervals for stability study can be executed as defined in the Stability Study Protocol based on agency/product or regulatory requirement (but not limited to).
Also read: SOP for Document Management System
-
-
Withdrawal of Stability Samples from Incubators:
-
-
- Withdraw stability samples (for all the conditions) from the stability chambers as on due date of withdrawal.
-
- In case if required withdrawal shall not be extended for +3 days (calendar) from the due date.
-
- The Stability Coordinator or designee shall make entries of the sample withdrawn in the “Stability Sample Reconciliation and Destruction Form” for “Qty. Drawn”, “Qty. Drawn Date / By”, “Station No./ Due on”, “Qty. Balance”, “Remarks” as per Annexure -10.
-
- The stability coordinator or designee shall withdraw the required sample quantity as specified in the Stability Study Protocol.
-
- Record the details of the sample quantity withdrawn in the reconciliation register (Annexure -10).
-
- Store the samples withdrawn for stability study under control room temperature conditions until the analysis and review.
-
- Note: Products which require some specific Temperature and relative humidity condition or highly sensitive to changing environmental condition shall be taken care as per the label claim or recommendation conditions and shall be stored in respective label claim condition.
-
-
Analysis of Stability Study Samples :
- The Stability Coordinator or designee shall issue the “Stability Template” for the stability sample analysis.
-
-
- Stability Coordinator or Designee shall make an entry in the Template/Protocol Issuance Register” as per SOP.
-
- The analyst shall put sign and date in the “Received By” column after receiving the analytical template.
-
- Section head should ensure that the analysis is performed as per the current version of stability specification as given in the stability testing templates.
-
- The analyst shall analyze the samples as per the current version of analytical test procedures and shall ensure the contemporaneous documentation.
-
- Complete the analysis within 25 calendar days from the withdrawal due date.
-
- Test which itself required more than 25 days for analysis then proper justification shall be given as per Annexure –11.
Also read: Process Validation SOP and Protocol
-
-
Reporting of Stability Study results :
-
-
- The analyst shall analyze the samples as per the test procedures given in the stability study Template. On completion of the analysis of all the tests, the analyst shall enter the results LIMS as well as in the Stability study summary report.
-
- However, the stability study summary report can be modified with respect to more information based on customer requirements.
-
- The analyst shall attach the chromatograms, UV spectrum, etc. with the template and shall submit the analytical along with the raw data for review to the designed person.
-
- After approval of the report, Stability study Summary Report ( Annexure–3 ) of the respective product /batch shall be updated.
-
- Any result, which is found out of specification or Out of trend, shall be intimated to the Head QC or designee immediately.
-
- Head-QC or designee along with Section Head shall investigate the Out of Specification (OOS) results according to the SOP “Investigation of Out of Specification Analysis Result”.
-
- In case of any stability study failure. Inform to Quality-Head. Quality – Head shall evaluate the data and for confirmation and shall discuss with a technical group comprising of Head-CQ, Head-Formulation, Head-FDD.
-
- Take the joint decision for Recall / Revision of formulation
-
- On completion of the stability study schedule of the product batch, the Head-Quality Control or designee shall give final conclusion and the report shall be submitted to the Quality-Head for the approval.
-
- On receipt of the approval, enter the data in the stability study protocol cum report (Summary Report) and file the documents in a stability file.
-
-
Significant Change during stability study:
-
-
- A 5% change in assay from its initial value or failure to meet the acceptance criteria for potency.
-
- Any degradation of products exceeding its acceptance criteria.
-
- Failure to meet the acceptance criteria for appearance, physical attributes and functionality test (e.g. color, phase separation, caking, hardness ) however some changes in physical attributes (e.g. softening of suppositories, melting of creams, disfiguration of capsules) may be expected under accelerated conditions and not to be considered as a significant change.
-
- As appropriate for the dosage form :
-
- Failure to meet the acceptance criteria for PH; or
-
- Failure to meet the acceptance criteria for dissolution for 12 dosage units.
-
- If “significant change “observed at 6 months testing of accelerated condition, then conduct additional testing at the intermediate storage condition up to 12 months, and the same shall be considered at the time of shelf-life evaluation.
-
- If significant change or failure of any attribute in one or more exhibit batches, intermediate storage condition study shall be performed for all three exhibit batches / all the test parameters of a particular pack.
Also read: Technology Transfer of Drug Product
-
-
Stability Schedule Verification:
-
-
- In-charge of the Stability section or designee shall prepare the monthly stability study schedule monitoring manually as well as in soft copy as per Annexure -6.
-
- Stability In-charge or designee shall update the soft copy report and manual Schedule as per requirements.
-
- At the end of the previous month, Stability In-charge or designee shall take the monthly Schedule from the computer with the help of filters.
-
- Stability In-charge or designee shall verify the computer monthly schedule against the manual schedule, make necessary corrections after proper verification with the master schedule for stability study.
-
- Stability of Sample Reconciliation & Destruction:
-
- The coordinator shall reconcile the stability samples after completion of analysis and shall make necessary entries for the destruction of the samples in “Stability Sample Reconciliation and Destruction Logbook” (Annexure-10).
-
- In addition to routine reconciliation check, periodic reconciliation of stock stability samples shall be performed by comparing the actual and recorded stocks (Six-month basis).
-
-
Discontinuation of study:
-
-
- Take the decision of discontinuation of stability studies on the basis of response received from FDD, QC Head, Quality Head on Non-conformity report or on the basis of APR.
-
- After the confirmation for discontinuation of stability study by Quality Assurance department, stability coordinator/reviewer shall filled the stability study discontinuation authorization form, mention the remaining stability study station or time point, remaining quantity to be destroyed, reason for destruction, destruction procedure to be followed, and Remarks if any, reviewed by QC Head and get authorization from the Quality Head as per Annexure-4.
-
- In case of discontinuation of stability study, the Stability study coordinator shall mention product name, batch no, stability condition, stability study station remained, the reason for discontinuation, reference, qty. to be destroyed and recommended in stability study discontinuation authorization form. (Annexure –4).
-
- Mention the reason for destruction. Follow the destruction procedure. remarks if any in the recommendations.
-
- Document the decision for stability study discontinuation, justified and approved by all authorized/ concerned persons.
Also visit: SOP for Control Sample Management
-
-
Handling of changes in specification or test procedure:
-
-
-
Condition 1: Specification changes but test procedure doesn’t change
-
-
- If next Stability study Station,
-
- Passes: Take Change control and revise the stability study protocol
-
- Fails: Trigger process change, keep stability samples with the new process, and continue old stability study with the old specification.
-
- Method validation: Not required if LOD, LOQ & linearity are established below the revised limit, Otherwise required.
-
-
Condition 2: Specification doesn’t change but tests procedure changes
-
-
- If the next stability study station with the validated method,
-
- Passes: Take Change control and revise the stability study protocol.
-
- Fail: Trigger process change, keep stability study samples with the new process, continue old stability with the old test procedure.
-
- Method validation: Required.
-
-
Condition 3: Specification, test procedure both changed
-
-
- If the next stability study station with the validated method,
-
- Passes: Take Change control and revise the stability study protocol.
-
- Fails: Trigger process change, keep stability study samples with a new process, and continue old stability study with old specification and test procedure
-
- Method validation: Required.
Stability Analysis Schedule and Cycle (Annexure – 1)
-
- Accelerated Stability study : 0, 3 and 6 months.
-
- Controlled Room Temperature Stability: 0,3,6,9,12,18,24,30,36,48,60 and 72 months. Depending upon the expiry period the stability study testing has to be continued for 12 months beyond the expiry.
-
- Intermediates: 0,3,6,9,12.( If necessary ).
-
- Maintain a stability study analysis schedule for each product as shown in Annexure-2
-
- Stability study shall be continued for one station beyond shelf life e.g; if the shelf life of the product is 24 months, stability study shall be carried upon 36 months.
Note: Stability samples (for all the conditions) shall be withdrawn from the stability study chambers as on due date of withdrawal. In case if required withdrawal shall not be extended for +3 days (calendar) from the due date.
Master Stability Schedule (Annexure – 2)
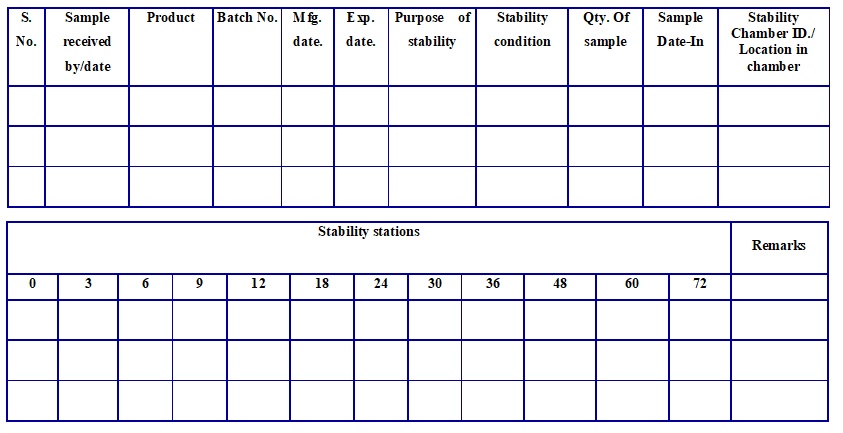
Summary (Stability) Report (Annexure – 3)
| Product Name: | ||||
| Protocol No.: | ||||
| Batch No.: | Mfg. date: | Exp. Date | ||
| Purpose of Study | ||||
| Packing Condition: | ||||
| Storage Condition: | Stability Study: (Accelerated, Long Term ) | |||
| ATP No./Specification No: | ||||
| Market | ||||
| Test | Specification | 0 Month | 3 Month | 6 Month | 9 Month | 12 Month |
Remark: Conclusion:
| Prepared By | Reviewed by | Approved By |
Stability Discontinuation Authorization Form (Annexure – 4)
| Product Name : | Batch No. : | |||
| Stability Condition : | ||||
| Stability station remained : | ||||
| Quantity to be disposed of: | Qty. Destroyed By/ Date | |||
| Reason for discontinuation:
Reference: |
||||
| Recommendation: | ||||
| Prepared By : | Date | : | ||
| Reviewed By : | Date | : | ||
| Authorized By : | Date | : | ||
Stability Sample Labels (Annexure – 5)
Monthly Schedule for Stability (Annexure – 6)
Monthly …………….. Year……….
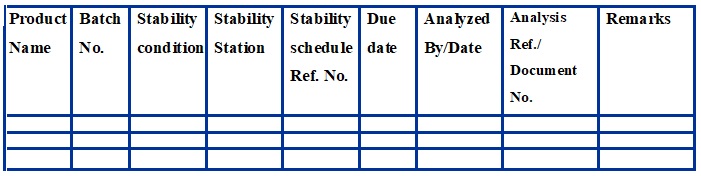
Example:-
A.R. No. is representing the report no. of stability analysis STB/ZZ/YMACC/XXXX;
STB/ZZ/YMLTC/XXXX (XXXX stand for Serial No. 0001, 0002,……, YM=1 Month,2Month,..;
ACC=Accelerated Climatic Condition; LTC=Long Term Condition; ZZ=Last two-digit of the year)
Stability Sample Quantity Format (Annexure – 7)
|
S. No. |
Product Name | Shelf Life (Months) | No. of Stations (ACC+LTC) | Required Quantity / Station |
Quantity to be Withdrawn (1.5 times of Qty / Station) |
||||
| A. | Sample for Chemical Analysis | ||||||||
| B. | Sample for Microbiological Analysis | ||||||||
Placebo Preparation Record (Annexure – 8)
Name of Product :
Reference BMR No. :
Placebo No. :
Date of Preparation :
Use before :
Quantity Prepared :
|
Sr. No |
Name of Material | A.R. No. | Quantity per tablet (mg) |
Quantity is taken (gm) |
Prepared by: Checked by: Approved by:
Date: Date: Date:
Placebo Record Book (Annexure – 9)
| S. No. | Name of Placebo | Date of Preparation | Use before Date | Code No. | Prepared by | Remarks |
Stability Sample Reconciliation and Destruction Form. (Annexure – 10)
Product :
B.No./Lot No. :
Quantity : ___________ Date in : __________
Condition :
|
Qty. withdrawal |
Qty. withdrawal | Station | Qty. Balance | Justification for extra sample requirement (If any) / Remarks |
Authorized by/date |
||
|
Date |
By | No. |
Due On |
||||
Extension Form For Stability Sample Analysis (Annexure – 11)
| SR.NO. | PRODUCT NAME | BATCH NO. | CONDITION | TEST |
JUSTIFICATION |



Pingback: Product Dossier Registration Process - Pharma Beginners
Pingback: Out of Specification Result in Microbiology - Guideline - Pharma Beginners
Pingback: Validation Master Plan (VMP) Preparation Guideline - Pharma Beginners
Pingback: SOP for BMR and BPR Review - Pharma Beginners
Pingback: Good Laboratory Practices (GLP) - SOP & Guideline - Pharma Beginners
Pingback: SOP for Out of Trend (OOT) Analytical Test Results - Pharma Beginners
Pingback: Stability Chamber - Operation, Cleaning and Performance check -
Pingback: Checklist for Review of Analytical Raw Data - Pharma Beginners
Pingback: Technology Transfer SOP of Drug Product - Pharma Beginners