Analytical Method Transfer (also called ‘Method Transfer’): A documented process that qualifies a laboratory (i.e., the Receiving Unit) to use an analytical test procedure that originated in another laboratory (i.e., the Transferring Unit), thus ensuring that the Receiving Unit has the procedural knowledge and ability to perform the transferred analytical procedure as intended
Analytical Method Transfer (USP 1224) Guideline / SOP
1.0 Objective :
-
- This SOP/Guideline describes the process and requirements for the transfer of analytical test methods from an originating laboratory (Transferring Unit) to a receiving laboratory (Receiving Unit).
-
- This SOP provides the requirements for the transfer of validated analytical methods.
-
- It defines a process for preparing for transfer, executing transfer testing and preparing final reports.
2.0 Scope :
-
- This Guideline describes the recommended steps to be taken to transfer validated analytical methods from the Transferring Unit (TU) Laboratory, which validated the methods to the Receiving Unit (RU) Laboratory, which accepts the methods and expects to perform analytical testing.
-
- This Guideline applies to quantitative and semi-quantitative (e.g. limit tests, etc.) analytical methods that are used to test pharmaceutical materials (raw materials), drug substances, intermediates, and/or ingredients and products that are critical in establishing the quality for the finished dosage form.
-
- In general, in-house test methods based on the following specialized techniques (but not limited to):
-
-
- HPLC,
-
-
-
- GC,
-
-
-
- AAS,
-
-
-
- UV,
-
-
-
- XRD,
-
-
-
- DSC,
-
-
-
- TLC,
-
-
-
- CE,
-
-
-
- Titrimetric,
-
-
-
- Microbiological Assay Methods,
-
-
-
- Particle Size Methods, shall be considered for Analytical Method Transfer.
-
-
- General procedures which lack specialized techniques like pH, Loss on Drying, Residue on Ignition, etc. and are commonly performed at the Receiving Unit (RU) need not be considered for However,
-
- If the laboratory receiving such analytical methods faces any issue(s) in the test method, it may request for a Analytical Method Transfer for the same from a laboratory that owns/uses the said test method.
3.0 : Workflow – Analytical Method Transfer :
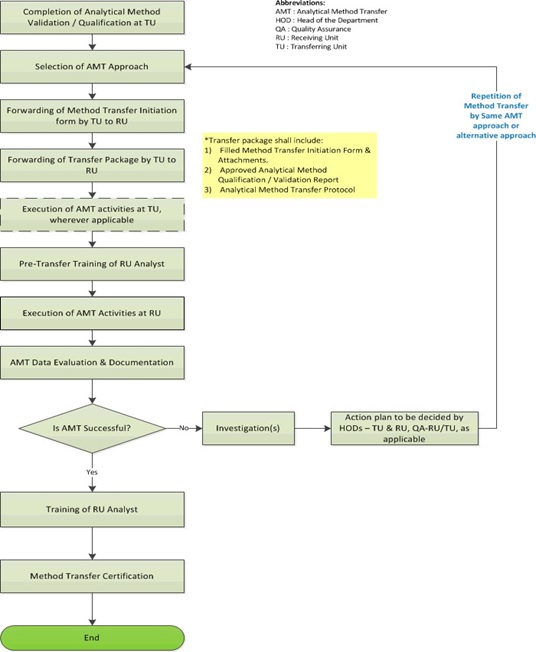
4.0 Process Description – Analytical Method Transfer:
-
-
Requirement:
-
-
- Individual method transfer protocols, with scientific justification and meaningful acceptance criteria shall be issued before the implementation of analytical method transfer.
-
- It is necessary for the Receiving Unit (RU) Laboratory to meet the predetermined acceptance criteria to assure that the RU Laboratory is qualified to run the procedure.
-
- Procedure(s) shall exist in both the Transferring Unit (TU) and RU Laboratories to ensure that any instruments/equipment used to validate/verify and transfer an analytical method have appropriate instruments /equipment qualification documentation.
-
- Analytical Procedure(s) shall exist in both the TU and RU Laboratories to ensure that any instruments/equipment’s used to validate/verify and transfer an analytical method are calibrated on a regular schedule and are within calibration intervals and specifications at time of use.
-
- Procedure(s) shall exist in both the TU and RU Laboratories to ensure that any required reference standards are properly qualified with supporting documentation available upon request.
-
- Procedure(s) shall exist in both the TU and RU Laboratories to evaluate discrepant laboratory data and results generated during either analytical method validation or transfer
-
- Analytical Procedure(s) shall exist in both the TU and RU Laboratories to ensure that training in the analytical technique(s) required by the analytical method(s) being transferred has been provided to analysts in both
-
-
Procedure(s) shall exist in the TU Laboratory to clearly outline the parameters and acceptance criteria for analytical methods validation.
-
-
- Analytical Method validation reports shall be made available to the RU Laboratory personnel.
-
- Care shall be taken to ensure that validated analytical methods can be successfully transferred to RU Laboratories and perform as validated.
-
- Analytical Procedure(s) shall exist in both the TU and RU Laboratories to detail the raw data recording, archival and retrieval procedures used by both laboratories.
-
-
Prior to drafting the analytical method transfer protocol,
-
-
-
- The RU Laboratory is provided with a copy of the method and method validation report (included in the Method Transfer Package).
-
-
-
- In the case of a newly validated method, the method validation report shall be approved by the validating laboratory management.
-
-
-
- QA of the validation site shall approve the validation report before the transfer protocol is approved and executed.
-
-
-
- It is the responsibility of the TU Laboratory to ensure that the RU Laboratory is supplied with a copy of the QA approved report.
-
-
-
- Any issues or concerns are to be resolved with the TU Laboratory prior to approval of the method transfer protocol.
-
-
- The TU Laboratory shall provide advance notification of critical equipment (e.g. instruments, computer data systems, etc.), safety requirements, Material Safety Data Sheet (MSDS) and reagents necessary for execution of the analytical method transfer.
-
- Release of commercial products cannot be performed until the method is properly transferred.
-
- Within expiry batches of commercial products shall not be used to support method transfer as it would create a compliance liability if an OOS result is encountered.
-
- Samples used for transfer can be samples from experimental batches, expired samples, spiked samples, samples rendered to be no longer representative of a commercial batch,
-
-
Analytical Method Transfer Protocol :
-
-
- The analytical method transfer process shall be protocol-driven, refer to Attachment II – Analytical Method Transfer (AMT) Protocol and Report (Example Template).
-
- The protocol shall contain the following details, at the minimum, but not limited to :
-
-
- Objective,
-
-
-
- Scope,
-
-
-
- Details of the analytical method transfer,
-
-
-
- Materials and instruments that will be used,
-
-
-
- Analytical procedure,
-
-
-
- Reference to method validation report,
-
-
-
- Experimental design,
-
-
-
- Acceptance criteria for all tests and/or methods included in the transfer,
-
-
-
- Deviation records (if any) and
-
-
-
- The final summary report including the test results.
-
-
- Based on the validation data and procedural knowledge, the transfer protocol shall identify the specific analytical performance characteristics (see USP Chapters <1225> and <1226>) that will be evaluated and the analysis that will be used to evaluate acceptable outcomes of the transfer exercise.
-
- The number of batches for which Analytical Method Transfer is to be performed shall be as per the following table:
Table A
| Sr. No. | Type of Product | Minimum No. of Batches# |
| 1. | API | One |
| 2. | Drug Product – Single Strength | One |
| 3. | Drug Product – Two or more than two strengths * | One Batch each – Lowest Strength & Highest Strength |
| * – An intermittent strength may also be included (in case of three or more than three strengths), in case of country / region-specific regulatory requirements, etc.
# – Country/region specific regulatory requirements for the number of batches required for Analytical Method Transfer shall also be considered, and indicated in the Site/Regional SOPs appropriately. |
||
-
INTRODUCTION TO ANALYTICAL METHOD TRANSFER:
-
- Method transfer can be accomplished by one of the following options (also see USP <1224>).
-
-
- Comparative testing with a set of pre-determined acceptance criteria.
-
-
-
- Co-validation between two laboratories.
-
-
-
- Complete or partial validation
-
-
-
- Experience with highly similar procedures or justification for transfer waiver.
-
-
- Analytical Method Transfer approach shall be selected based on criticality or complexity of test method, facilities available at TU and RU, requirements of applicable regulatory agencies, etc.
-
COMPARATIVE TESTING APPROACH:
- Pre-Transfer Activities:
-
- Based on requirements for analysis of new drug products/API/API Intermediates/Key Raw Materials for API (as applicable) at specific locations, the concerned laboratory at the location (hereinafter referred to as ‘RU’) and the laboratory which owns or is using the said analytical method(s) (hereinafter referred to as ‘TU’), shall identify the need for Analytical Method Transfer.
-
- Once a need for Analytical Method Transfer is determined, a Method Transfer Initiation Form (refer to Attachment I) shall be sent by the TU to RU for information and intimation Form.
-
-
The following minimum details shall be attached along with the Method Transfer Initiation Form:
-
-
-
- Test Method(s) to be transferred
-
-
-
- Test Method(s) to be excluded from Analytical Method Transfer with justification based on criticality of procedure and complexity of analysis
-
-
-
- List of Instruments/Equipment required during Analytical Method Transfer (with name of Manufacturer)
-
-
-
- MSDS (for Actives)
-
-
-
- List of Reagents & chemicals/solvents
-
-
-
- HPLC/GC Columns (with name of Manufacturer)
-
-
-
An example template for Method Transfer Initiation Form is given as Attachment I
-
-
- Upon receipt of the Method Transfer Initiation Form, RU HOD (or designee) shall evaluate the RU’s preparedness for Method Transfer and make a decision regarding the acceptance or rejection of the Method Transfer.
-
- If the Method Transfer is accepted, RU HOD (or designee) shall send the Method Transfer Initiation Form duly signed to the TU HOD (or designee).
-
- Incase the Method Transfer is not accepted, RU HOD shall provide the reason for not accepting the Method Transfer and provide an alternate plan.
-
- If the RU needs support from the TU for arrangement of columns, reagents, working standards, reference standards, placebo, etc.,
-
- The RU HOD (or designee) may communicate this to the TU HOD (or designee) and seek TU’s support.
-
- Before Method Transfer is initiated at the site, the required supporting documents shall be compiled by the TU and such a compilation is called the Transfer Package.
-
-
The Transfer Package shall include the following (but not limited to):
-
-
-
- Filled-out Method Transfer Initiation Form and its attachments as listed above.
-
-
-
- Approved Analytical Method Qualification Validation / Verification report.
-
-
-
- Analytical Method Transfer Protocol duly signed.
-
-
- Method Transfer activities shall be initiated at RU only after receipt of the Transfer Package from the Additionally, the Analytical Method Transfer Protocol should be signed off by all relevant stakeholders of the TU and RU before initiation of Method Transfer activity.
-
- All Method Transfer documents shall be retained for the retention period as specified in the site retention policies.
-
- Analytical Method Transfer Protocol:
-
- The TU HOD (or designee) and the RU HOD (or designee), in consultation with each other, shall make a decision about the type of transfer to be conducted based on criticality or complexity of test method, facilities available at TU and RU, requirements of applicable regulatory agencies, etc.
-
- Accordingly, the Analyst – TU shall prepare an Analytical Method Transfer Protocol and assign it a unique number.
-
-
The Analytical Method Transfer Protocol shall have the following minimum details (as applicable):
-
-
-
- Pre-Approval Signature Records
-
-
-
- Objective and Scope/Product Details
-
-
-
- Analytical Method Transfer and Test Details:
-
-
-
- Type of Analytical Method Transfer
-
-
-
- Name and Location of TU
-
-
-
- Name and Location of RU
-
-
-
- Test(s) intended to be transferred
-
-
-
- STP No. (Along with Version No.)Specification No. (Along with Version No.),
-
-
-
- Method Validation/Qualification / Verification report
-
-
-
- Analytical Method Transfer Plan and Acceptance Criteria.
-
-
-
- Details of Working/Reference Standards
-
-
-
- Details of Reference Samples Used for Analytical Method Transfer
-
-
-
- Placebos Details used for Analytical Method Transfer
-
-
-
- Impurities Details of used for Analytical Method Transfer
-
-
-
- Details of Instruments/Equipment used for Analytical Method Transfer
-
-
-
- Analytical Method Transfer Results
-
-
-
- Deviation Record
-
-
-
- Training Record
-
-
-
- Analytical Method Transfer Final Summary/ Report
-
-
-
- Post-Approval Signature Records
-
-
- The Analytical Method Transfer Protocol, after preparation by TU Analyst and review/approval from relevant stakeholders of TU (TU HOD/designee, etc.) shall be sent for review to relevant stakeholders of RU.
-
- The Analytical Method Transfer Protocol shall then be reviewed/approved by relevant personnel within RU (HOD/designee – RU, QA – RU, etc.).
-
- If the Analytical Method Transfer Protocol has issues that need to be resolved before approval, this shall be communicated to TU and appropriate corrections shall be initiated by TU.
-
-
The Analytical Method Transfer Protocol shall be pre-approved by at least the following:
-
-
-
- HOD (or designee) – TU
-
-
-
- QA – RU
-
-
-
- HOD (or designee) – RU
-
-
- The Analytical Method Transfer Protocol shall be ready for implementation once approval signatures of both RU and TU stakeholders are completed.
-
- Analytical methods shall be transferred as per the preapproved Protocol.
-
-
Analytical Method Transfer by Comparative Testing – General Process Highlights:
-
-
- Method Transfer shall be done with current version of approved or effective Standard Test Procedure (STP), as applicable.
-
- Only validated / qualified / verified analytical methods shall be transferred from TU to RU and the transfer shall be executed based on pre-approved Protocol.
-
- Samples selected for Method Transfer shall meet the specification for the test(s) required to be transferred, but shall not be of product in commerce.
-
- The RU shall ensure availability of all reagents/chemicals, equipment, working standards, reference standards, placebos, trained manpower, etc. required for Analytical Method Transfer.
-
- The equipment used by RU during Method Transfer should meet appropriate specifications to ensure the requirements of the method.
-
- Whenever there is an update in an STP due to a change in a method(s) (e.g., due to Pharmacopoeial change), the change control request evaluation must include an evaluation of whether the proposed change in the method will require repetition of the Analytical Method Transfer.
-
-
If such an evaluation of a STP change mandates the need for the Analytical Method Transfer to be repeated,
-
-
- Then a transfer for that particular method shall be performed for the revised version of the STP.
-
- Analysis of samples using the revised STP shall start only after completion of a Method Transfer.
-
- In this condition, Analytical Method Transfer needs to be repeated only for the method(s) that has undergone the change.
-
- A scenario in which an STP is revised after an Analytical Method Transfer Protocol has been approved for a particular version of the STP, shall be handled as under:
-
- If the STP revision mandates the need for Analytical Method Transfer Protocol revision, the Protocol shall be reviewed and revised as per applicable documentation control procedures and Analytical Method Transfer shall be carried out based on the revised version of the STP.
-
- If the STP revision does not require Analytical Method Transfer Protocol revision, a planned deviation may be raised as per applicable local procedures at RU and Analytical Method Transfer may be completed with the previous version of the Analytical Method Transfer Protocol.
Note: This condition shall apply only when the changes are such that they do not affect the analytical methods under transfer.
-
- If the tests for Assay and Content Uniformity (CU) are performed using the same method, RU and TU may opt to perform Analytical Method Transfer for either one of them
-
- All impurities/solvents present in the method must be spiked for confirmation of RT/RRT(s) in the test methods for impurities/solvents.
-
- For qualitative and quantitative microbial tests like BET, Sterility Test and MLT (also called as Microbial Enumeration Tests and Tests for Specified Micro-organisms),
-
- An onsite validation approach shall be followed to establish the methods at RU; Analytical Method Transfer approach is not recommended.
-
-
RU may use Validation Protocol of TU for the same, if available.
-
-
- Tests for Microbiological Assay may, however, be validated at TU and, thereafter, transferred by any of the Analytical Method Transfer approaches to RU.
-
- Method Transfer is not needed to be performed for those tests which are being validated at the RU.
-
- Sample analysis as per a method under transfer shall only be started after the applicable Method Transfer Certificate has been issued.
-
- The TU shall ensure that samples sensitive to moisture, light or temperature are appropriately packed to maintain integrity before they are sent to RU.
-
- Any deviation from pre-approved Analytical Method Transfer Protocol arising prior to, or during Analytical Method Transfer exercise at TU shall be handled as per relevant procedures at TU and approved by (but not limited to): HOD/designee – TU and QA – TU.
-
-
This shall be documented in the Analytical Method Transfer Report.
-
-
- Any deviation from pre-approved Analytical Method Transfer Protocol arising prior to, or during Analytical Method Transfer exercise at RU shall be handled as per relevant procedures at RU and approved by (but not limited to):
-
- HOD/designee – TU, HOD/designee – RU and QA – RU and this shall be documented in the Analytical Method Transfer Report.
-
- A general list of tests for API and Drug Products that need an Analytical Method Transfer to be performed is given in Section 1.
-
- Important Note: However, STPs and country/region specific regulatory requirements also need to be considered for the same.
Section-1
-
-
Active Pharmaceuticals Ingredients (API) –
- Assay,
-
-
-
- Impurities/Trace Metal Estimation/ Residual Solvent,
-
-
-
- Particle Size
-
-
-
Solid Dosage (Drug Product) :
-
-
-
- Assay,
-
-
-
- Content Uniformity,
-
-
-
- Impurities/Trace Metal Estimation/ Residual Solvent,
-
-
-
- Dissolution, Preservative/ Anti-oxidant/ other additives in Drug Product.
-
-
-
Parenteral (Drug Product) :
-
-
-
- Assay,
-
-
-
- Content Uniformity,
-
-
-
- Impurities/Trace Metal Estimation/ Residual Solvent, Preservative/ Anti-oxidant/ other additives in Drug Product.
-
-
-
Ointments/ Creams (Drug Product) :
-
-
-
- Assay,
-
-
-
- Content Uniformity,
-
-
-
- Impurities/Trace Metal Estimation/ Residual Solvent,
-
-
-
- Preservative/ Anti-oxidant/ other additives in Drug Product.
-
-
-
Liquids/Suspension (Drug Product) :
-
-
-
- Assay,
-
-
-
- Content Uniformity,
-
-
-
- Impurities/Trace Metal Estimation/ Residual Solvent,
-
-
-
- Dissolution (wherever applicable),
-
-
-
- Preservative/ Anti-oxidant/ other additives in Drug Product
-
-
-
Ophthalmic (Drug Product) :
-
-
-
- Assay,
-
-
-
- Content Uniformity,
-
-
-
- Impurities/Trace Metal Estimation/ Residual Solvent,
-
-
-
- Preservative/ Anti-oxidant/ other additives in Drug Product
-
-
-
Comparative Testing may be further classified as:
-
-
-
- Collaborative (also called Indirect Analytical Method Transfer).
-
-
-
- Non-Collaborative (also called Direct Analytical Method Transfer).
-
-
-
- Collaborative Type of Method Transfer (Indirect Method Transfer):
-
-
- The TU Analyst shall first analyze reference samples of each batch at TU laboratory six times.
-
- However, for CU test, 10 units, and for dissolution test, six units shall be analyzed for each batch.
-
- In case of particle size test methods, analysis shall be performed in triplicate.
-
- In case of limit tests, single analysis of reference samples of each batch shall be done.
-
- The RU Analyst shall then independently perform the same analysis at the RU laboratory using the same ( * ) reference standards, experimental procedures, and reference samples of each batch as used by the TU Analyst in the TU laboratory.
-
- Analysis shall be performed six times for reference sample of each batch.
-
- However, for CU test, 10 units, and for dissolution test, six units shall be analyzed.
-
- In case of particle size test methods, analysis shall be performed in triplicate.
-
- In case of limit tests, single analysis of reference samples of each batch shall be done.
-
- The time between the analyses at the two locations for Method Transfer shall not be more than 30 working days (from time of testing initiation at either site till testing completion at both sites).
-
- Any deviation from this time frame shall be justified and documented as per the written procedures as per SOP : Handling of Deviations/Incidents).
-
-
Non-Collaborative Method Transfer (Direct Method Transfer):
- This type of Method Transfer may be done with either of the two approaches as shown below:
-
-
-
- Three-Way Approach
-
-
-
- Two-Way Approach
-
Note: The type of approach shall be selected based on the type of product, test method complexity/criticality, regulatory requirements, etc.
-
-
Three-Way Approach:
-
-
- In this type of approach, reference samples of each batch shall be analyzed initially in the TU laboratory.
-
- The supporting analytical data along with chromatograms shall be provided to RU.
-
- Thereafter, preferably, the same TU Analyst (or any other TU Analyst) shall demonstrate the method to the RU Analyst by analyzing reference samples of the same batch (es) at the RU laboratory.
-
- The RU Analyst shall repeat the same analysis using reference samples of the same batch (es) independently in the presence of the TU Analyst.
Note:
-
- In case of test for CU, 10 units of reference samples of each batch shall be tested at TU laboratory by TU Analyst.
-
- Thereafter, 10 units, of the reference samples of the same batch (es) shall be tested at RU laboratory by TU Analyst followed by RU Analyst also testing 10 units of the reference samples of the same batch (es).
-
- In case of dissolution test, six units are to be tested at TU laboratory by TU Analyst.
-
- Thereafter, six units of the reference samples of the same batch (es) shall be tested at RU laboratory by TU Analyst followed by RU Analyst also testing six units of the reference samples of the same batch (es).
-
- In case of particle size test, the TU Analyst shall first perform the analysis at TU laboratory in triplicate.
-
- Thereafter, the reference samples of the same batch (es) shall be tested in triplicate at RU laboratory by the TU Analyst followed by the RU Analyst also performing this test on the reference samples of the same batch (es) in triplicate.
-
- In case of limit tests, single analysis shall be done by the TU Analyst at the TU laboratory.
-
- Thereafter, reference samples of the same batch (es) shall be tested at RU laboratory once each by the TU Analyst and the RU Analyst.
-
-
Two-Way Approach:
-
-
- In this type of approach, the TU Analyst shall analyze samples at the RU laboratory.
-
- The RU Analyst shall repeat the same analysis on reference samples of the same batch (es) independently in the presence of the TU Analyst.
Note:
-
- In case of test for CU, 10 units of reference samples of each batch shall be tested at RU laboratory by TU Analyst followed by RU Analyst also testing 10 units of the reference samples of the same batch (es).
-
- In case of dissolution test, six units of the reference samples of each batch shall be tested at RU laboratory by TU Analyst followed by the RU Analyst also testing six units of the reference samples of the same batch (es).
-
- Particle size test, reference samples of each batch shall be tested at the RU laboratory by the TU Analyst in triplicate followed by the RU Analyst also performing this test on the reference samples of the same batch (es) in triplicate.
-
- In case of limit tests, reference samples of same batch (es) shall be tested once each by the TU Analyst and the RU Analyst at RU laboratory.
-
-
Training During Method Transfer:
-
-
- Before initiation of the Analytical Method Transfer, the RU Analyst shall be trained on the test method.
-
- This training shall be documented in the Analytical Method Transfer Report.
-
- Once a method has been successfully transferred to a respective group at the RU, relevant groups of the RU shall be trained by either the TU Analyst (wherever possible) or the RU Analyst and recorded in the Analytical Method Transfer Report.
-
- Thereafter, the RU Analyst shall follow a respective ‘On-the- Job Training’ system in order to train other Analysts.
-
- Analytical Method Transfer is not needed to be repeated to train other Analysts at the RU.
-
-
Data Evaluation and Documentation:
-
-
- Analyst – TU and Analyst – RU shall compile and evaluate the test results generated during Analytical Method Transfer.
-
- These results shall be further reviewed and approved by (but not limited to): HOD/designee – RU and QA – RU.
-
- If the data complies with the acceptance criteria as detailed in the SOP (refer to Annexure-4) a ‘Method Transfer Certificate’ shall be prepared by TU and RU Analysts.
-
-
This Certificate shall be further reviewed and approved by the following (but not limited to):
-
-
-
- HOD/designee – RU
-
-
-
- QA – RU
-
-
- Original copy of approved Method Transfer Certificate (refer to Annexure-4) shall be retained at the RU.
-
- Original raw data, worksheets, chromatograms, calculations, results, etc., along with data generated at the TU shall be archived at the RU.
-
- A copy of the Method Transfer Certificate along with raw data, worksheets, chromatograms, calculations, results, etc., shall also be archived at the TU.
-
- All invalid chromatograms and raw data generated as results of experimental failure shall also be archived along with the Method Transfer raw data and the reason for making them ‘invalid’ shall be mentioned and approved by relevant stakeholders.
-
- If the acceptance criteria are not met in case of Method Transfer, a laboratory investigation shall be performed in consultation with QA-TU and QA-RU to find out an assignable cause as per prevalent procedures at RU.
-
-
The laboratory investigation shall include the following parameters at minimum (as applicable):
-
-
-
- Interview of analyst to confirm analyst knowledge,
-
-
-
- Performance of instruments/equipment used,
-
-
-
- Evaluation of calculation associated with testing,
-
-
-
- Review of Analytical Method Transfer Report,
-
-
-
- Acceptance criteria, etc.
-
-
- As a part of investigations, the TU may also need to review the initial analytical method validation / qualification / verification reports again and if required,
-
- The analytical method may be re-validated / re-qualified / re-verified at TU before repeating the Analytical Method Transfer.
-
- Based on the investigation conclusion, a further action plan shall be decided by HOD/designee-TU, HOD/designee-RU and QA-TU/RU (as applicable).
-
- If justifiable, the investigation conclusion may also indicate the need to change the type of Analytical Method Transfer approach and suggest a repeat of Analytical Method Transfer with the alternate approach.
-
- All investigation reports shall be filed along with the other Method Transfer documents (refer to the current version of SOP for – Investigations).
-
-
In case of any other deviation from written procedure/Protocol during Analytical Method Transfer,
-
-
- A deviation report shall be prepared. Such deviations shall be handled as per prevalent procedures at TU / RU, as applicable.
-
- Irrespective of the test results, a Method Transfer Certificate shall be prepared by TU and RU Analysts.
-
- An example template for the same is given as Annexure-4.
-
- Once Analytical Method Transfer data evaluation and summarization has been completed,
-
- The Analytical Method Transfer Report, Method Transfer Certificate and other associated documents shall be sent for review and approval to the following:
-
-
- QA – RU
-
-
-
- HOD (or designee) – RU
-
Note: A clear statement whether the Method Transfer has been successfully or unsuccessfully completed shall be included in the Analytical Method Transfer Report, as well as in the Method Transfer Certificate.
-
Validation Approach:
- Partial or complete validation of analytical method may be considered as an alternate approach for Method Transfer in one or more of the following cases (but not limited to):
-
-
- Co-validation approach chosen by the TU and RU where the RU provides data for the assessment of reproducibility.
-
-
-
- Applicable regulatory agency requirements for the same.
-
-
- Performing Analytical Method Transfer is not feasible due to the following (but not limited to):
-
-
- Less stable molecule or Drug Product / Drug Substance being too sensitive.
-
-
-
- Drug Product / Drug Substance being a narcotic/ psychotropic substance; hence, legal complications in transporting the same.
-
-
- Considerable difference in the technical characteristics of test equipment available at RU as compared to that of TU.
-
- Validation approach shall be initiated at the RU after agreement of the same by HOD (or designee) – TU and HOD (or designee) – RU (refer to the SOP – Analytical Method Validation.
-
- Whenever ‘Partial Validation’ is being used as an alternate for Analytical Method Transfer,
-
-
The following minimum parameters shall be included:
-
-
-
- Accuracy
-
-
-
- Linearity
-
-
-
- Specificity
-
-
-
- Method Precision
-
-
-
- Confirmation of LOD and LOQ of impurities/solvents
-
-
-
- Confirmation of RT/RRT of impurities/solvents
-
Note: The re-validated method at RU shall be considered as the final method.
-
- A Protocol for Validation shall be prepared at the RU and shall be reviewed and approved by the HOD (or designee) – RU, HOD (or designee) – TU and QA – RU based on existing Analytical Method Validation Protocol.
-
- The acceptance criteria for this shall be as in the relevant Method Validation SOPs and existing Analytical Method Applicable regulatory agency requirements for the same.
-
Analytical Method Transfer Waiver:
- Analytical Method Transfer may be waived under certain circumstances.
-
- In such a case, the RU is considered to be qualified to use the analytical test procedure without the need for Analytical Method Transfer by comparative testing or validation approaches.
-
- Method Transfer waiver shall be initiated at the TU after agreement of the same by HOD (or designee) – TU and HOD (or designee) – RU.
-
-
Waiver of Method Transfer may be considered under one or more of the following conditions (but not limited to):
-
-
-
- The analytical procedure is the same or very similar to a procedure already in use at
-
-
-
- The new dosage form possesses either a comparable composition or concentration of API relative to an existing product.
-
-
-
- The new methods involve changes that do not substantially alter the ability to use the method (e.g., changes in sample preparation procedures, concentration or changes in calculation formulas).
-
-
-
- Changes made in manufacturing process of API, which do not impact existing analytical procedure.
-
-
-
- Changes made in API source or recipe of drug product, which do not impact existing analytical procedure.
-
-
-
- The method is in use at RU for a long time, but Analytical Method Transfer was not performed previously.
-
-
-
- This condition may be considered only if there has been no history of inconsistencies/failures.
-
-
-
- A example template for ‘Method Transfer Waiver Certificate’ is given as Annexure-5.
-
-
- The reason/justification for not performing Method Transfer shall be appropriately documented.
-
Planning – Analytical Method Transfer
-
- The management of Transferring laboratory and Receiving laboratory designate personnel if required from each laboratory to execute and document method transfer.
-
- Transferring laboratory shall procure and analyze the evaluation sample from formulation development department /Organic synthesis department/Plant and fill those results in method transfer protocol.
-
- Transferring laboratory shall prepare the analytical method transfer protocol/report (refer Annexure -2) as per acceptance criteria given in Annexure-3.
-
- As mentioned in Annexure-2, evaluation sample shall be analyzed in triplicate for each test (Other than Content uniformity and Dissolution) and mean value to be reported in protocol.
-
- Register for method transfer protocol/report entry shall be maintained at Transferring laboratory.
-
- Transferring laboratory shall send the analytical method transfer protocol/report, evaluation sample, approved specification and ATP to Receiving laboratory.
-
- Receiving laboratory should store the evaluation sample as per recommended storage condition to avoid differences in result due to storage conditions/ period.
-
-
Receiving laboratory shall ensure the availability of following, but not limited to, requirements for method transfer.
-
-
- Approved Specification and Test procedure from R&D.
-
- Approved Analytical Method Transfer protocol/report from R&D.
-
- Analytical method validation report.
-
- Reference standard, Impurity standard along with COA.
-
- Sample for evaluation.
-
- Equivalent instruments, columns, reagent etc. as specified in method.
-
- MSDS for Drug substances as well as Drug product
-
Template preparation and Issuance procedure
-
- Based on Analytical method transfer protocol/report Section-head or designee shall make entry for start date (Date of receipt of protocol) in method Transfer template register (refer Annexure -8).
-
- Based on Analytical method transfer protocol/report section-head or designee shall prepare Template to record raw data including, but not limited to the following.
-
-
- Title of method transfer with test details for drug substance.
-
-
-
- Reference analytical test procedure number and version, protocol no, method validation report no.
-
-
-
- Name of the instruments/equipments.
-
-
-
- Name of reagents /chemical to be used with make.
-
-
-
- Working standard /impurity standard with lot No. /potency and date before use.
-
-
-
- Study design with acceptance criteria and provision to record raw data.
-
-
-
- Calculation formula.
-
-
-
- Provision to record results, mean, RSD and other related values.
-
-
Execution of Analytical Method Transfer
-
- Head-QC or designee of receiving laboratory shall request to Transferring laboratory for deputing their analyst to conduct training, demonstrate and assist in analysis, if required.
-
- Section head or designee shall impart the training at least to analyst who are involved in the AMT.
-
- Section-head or designee of Receiving laboratory shall instruct to analyst to read carefully about method transfer template or concerned SOP before performing the method transfer activity.
-
- Receiving laboratory Analyst shall perform analysis as per approved template, and record raw data.
-
- Analyst shall be sign after each preparation.
-
- Analyst shall compile the observations / discrepancies and compare the result with R&D results as per acceptance criteria given in analytical method transfer template.
-
- Write any deviations from protocol /observations / discrepancies observed during method transfer in space provided in analytical method transfer protocol/report and report to Section-head or designee and take necessary authorization.
-
-
Analyst shall fill the results obtained in space provided for Receiving laboratory in analytical method transfer protocol/report.
-
-
- Section-head or designee of Receiving laboratory shall check and ensure the result of method transfer are met as per acceptance criteria mentioned in analytical method transfer protocol/report, conclude, mention remarks if any and inform to Head-QC or designee.
-
- If results of method transfer complies as per acceptance criteria, method transfer shall be considered as complete, after the document is signed off by both transferring unit and receiving unit.
-
- In final conclusion the receiving unit is now qualified to run the procedure should be mentioned by section head of receiving unit.
-
- In case of non-compliance of acceptance criteria, deviations from protocol the analysis of both the end shall be investigated as per current version of SOP of handling of repeat analysis
-
- Concluded in the analytical method transfer protocol/report.
-
- Corrective and preventive actions decided based on deviations from protocol should be mentioned in the protocol/report.
-
- During method transfer if there is change in method then impact on validation needs to be evaluated
-
- If required, transferring unit needs to validate impacted method parameters again and then method transfer shall be started.
-
-
Head-QC or designee of Receiving laboratory shall verify the conclusion.
-
-
- Specification and Standard test procedure shall be revised based on the method transferred protocol if required.
-
- All the concerned persons shall sign on the executed protocol, which shall be considered method transfer report ( refer Annexure -2).
-
- After approval of executed protocol, Receiving laboratory shall strike off protocol in title and copy of method transfer report shall be sent to Transferring laboratory for archival.
-
- Date on which the copy of method transfer report was sent to Transferring laboratory for archival shall be entered as “Completed date” in method transfer template register. (Annexure-7)
-
- In case acceptance criteria are not met and based on observations/ discrepancies, Transferring laboratory shall revise the method and send the revised copy of method/Analytical test procedure and justification if necessary to Receiving laboratory.
-
- In case of any failure, Head -QC or designee of Receiving laboratory shall evaluate and decide further course of action.
-
Handling Analytical testing issues after successful Analytical Method Transfer or Method Transfer waiver:
- After successful Analytical Method Transfer or Method Transfer Waiver, if inconsistencies/failures are observed during testing, the RU shall initiate investigations.
-
- The investigation shall also include review of Analytical Method Transfer Report and other similar OOS / Failure Investigations.
-
- If the investigation reveals ‘test method’ as the probable root-cause,
-
- Then, the investigation report shall be shared with the TU with a request for repeat of Analytical Method Transfer or analytical method validation, as applicable.
5.0 ANNEXURES:
Annexure-1- Method Transfer Initiation Form “Template”
|
ANALYTICAL METHOD TRANSFER INITIATION |
|
| From (TU): | To (RU): |
| Name of API/Product:
STP No. of the Test Method to be transferred: |
|
| The following items are being attached (‘ √ ’) shows item attached):
Test Methods to be transferred Test Methods to be excluded with justification based on criticality of procedure and complexity of analysis List of Instruments/Equipment required (with name of Manufacturer) MSDS (Actives) List of Columns (with name of Manufacturer) List of Working Standards/Reference Standards List of Reagents Types of Filters |
|
| Form Prepared By (TU): | Approved By (TU-HOD or designee): |
| Received By (RU) (Signature/Date): | |
| Initiate Method Transfer? (to be filled by RU)
Yes No (Provide Reason with Alternate Plan) Data Filled By (Sign/Date): Approved By (RU-HOD or designee) (Sign/Date): |
|
Annexure-2- Analytical Method Transfer (AMT) Protocol and Report
| Sr. No. | Title |
| 1.0 | Pre-Approval |
| 2.0 | Objective and Scope |
| 3.0 | AMT Details |
| 4.0 | Test(s), Test Method(s) & Specification Limits |
| 5.0 | AMT Study Plan |
| 6.0 | Details of Working Standards/Reference Standards |
| 7.0 | Details of Batches Used For AMT |
| 8.0 | Details of Placebo(s) Used For AMT |
| 9.0 | Details of Impurities Used For AMT |
| 10.0 | Details of Instrument(s)/Equipment Used for AMT |
| 11.0 | AMT Results |
| 12.0 | Deviation Record |
| 13.0 | Training Record |
| 14.0 | Final Summary Report |
| 15.0 | Reference Documents |
| 16.0 | Abbreviations |
| 17.0 | Attachments |
| 18.0 | Post-Approval |
1.0 PRE-APPROVAL
The ‘Prepared By’ signature indicates that this document has been prepared in accordance with existing cGxP standards and adequately reflects the tasks and deliverables necessary for transfer of the said analytical method.
| Prepared by/ Function | Designation | Signature | Date |
The ‘Reviewed and Approved By’ signatures indicate that, this document has been reviewed and it adequately reflects the tasks and deliverables necessary for transfer of the said analytical method and that the documentation and information included herein complies with applicable regulatory, corporate, divisional / departmental and cGxP requirements.
| Reviewed & Approved By/ Function | Designation | Signature | Date |
2.0 OBJECTIVE AND SCOPE
(This section shall be filled before the protocol is pre-approved)
The objective and scope of this document is to verify and document the transfer of the (test method(s), STP No.) for (Product Name) from (name of TU) to (name of RU).
Successful completion of this activity as defined in this document will provide documented evidence that the method has been transferred in accordance with the defined and documented specifications and acceptance criteria.
3.0 AMT DETAILS
(This section shall be filled before the protocol is pre-approved)
| Type of Method Transfer
(mark the applicable check box) |
Collaborative:
Non-Collaborative (Direct) : |
| Transferring Unit (Name and Location) | |
| Receiving Unit (Name and Location) |
4.0 TEST DETAILS
(This section shall be filled before the protocol is pre-approved)
| Tests Intended To Be Transferred | Test Method (STP) No. | Specification No. | Specification Limits | Method Validation/Verification Report No. (if applicable) |
5.0 ANALYTICAL METHOD TRANSFER STUDY PLAN
(This section shall be filled before the protocol is pre-approved)
| Parameter | Test Plan Acceptance | Acceptance Criteria |
6.0 DETAILS OF WORKING STANDARD(S)/REFERENCE STANDARD(S)
(This section shall be filled during AMT execution)
| Standard
Name(s) |
Batch No(s) | Manufacturer | Validity | Potency |
7.0 DETAILS OF BATCHES USED FOR AMT
(This section shall be filled at the time of AMT execution and should include a brief justification for the samples chosen, including any specific dosage strengths. For examples: stressed samples, impurity spiked samples, high and low dosage strengths, etc. appropriate to demonstrate the successful application of the method by both laboratories.)
| Batch No(s). | Strength | Expiry Date |
8.0 DETAILS OF PLACEBOS USED FOR AMT
(This section shall be filled during AMT execution)
| Batch No(s). | Strength | Manufacturing Date | Validity |
9.0 DETAILS OF IMPURITIES USED FOR AMT
(This section shall be filled during AMT execution)
| Impurity name | Batch No(s). | Validity |
10.0 DETAILS OF INSTRUMENT(S)/EQUIPMENT USED FOR METHOD TRANSFER
(This section shall be filled during AMT execution)
| Instrument or Equipment Name / Location | ID /Code | Instrument Make | Calibration due date |
11.0 AMT RESULTS
(This section shall be filled during AMT execution; relevant table to be selected based on type of approach)
Table A
(To be used in case of collaborative type of method transfer)
| Parameter | Acceptance Criteria | Result At TU | Result At RU | Comparison of Results at TU/RU | Passes Acceptance Criteria? ( Yes/No) |
(Raw Data, Chromatograms, etc. shall be attached to this Report)
Table B
(To be used in case of non-collaborative type of method transfer – 2 way approach)
| Parameter | Acceptance Criteria | Result At RU (For Analysis done by TU Analyst) | Result At RU (For Analysis done by RU Analyst) | Comparison of Results | Passes Acceptance Criteria?
( Yes/No) |
12.0 DEVIATION RECORD
(This section shall be filled during AMT execution)
| Deviation Number | Description of Deviation |
(Deviation details may be attached as supporting documents.)
13.0 TRAINING RECORD
(This section shall be filled during AMT execution – applicable training shall be chosen based on the options given below)
- Pre-AMT Training – RU Analyst
| Training Subject: | ||
| Training Method (tick the applicable option) | Self-Reading | Instructor Led |
| Name | Sign & Date | |
| Training Received By (RU Analyst (s)) | ||
| Training Imparted By (TU Analyst, etc.)
(in case of Instructor Led Training) |
||
Post-AMT Training to RU
| Training Subject: | ||
| Training Method
(tick the applicable option) |
Self-Reading | Instructor Led |
| Name | Sign & Date | |
| Training Received By (RU Analyst (s)) | ||
14.0 FINAL SUMMARY / REPORT (This section shall be filled post- AMT execution)
- Analysis / Evaluation of Data & Summary——————————————————————–
- Conclusion
Based on evaluation of data against acceptance criteria, methods of _______________ was/were found to be:
- Transferred successfully
- Not transferred successfully
Comments (if any)
Annexure-3- Acceptance Criteria for Analytical Method Transfer (AMT)
Refer to this link :. Acceptance Criteria for AMT
Annexure-4- Method Transfer Certificate
- Certificate No. / AMT Protocol No.:
- Product Name:
- Receiving Unit:
- STP No.:
- SOP No.:
- Test Data: (To be given as an attachment)
- Data Analysis: (To be given as an attachment)
- Has requisite training been completed? Yes No
- Conclusion:
Method Transfer study for _________________ has been completed as per Protocol No.__________ using the current approved/effective STP No.____________.
Upon review of the data collected, no methodology problems occurred.
The acceptance criteria were met. The method(s) for ______________ is/are considered officially transferred upon certificate approval.
OR
The method (s) for ______________has/have not been transferred successfully.
The observations made in the method while the transfer exercise was underway are given as an attachment.
Refer to the attached copies of laboratory investigations and/or deviations (if any).
| PREPARED BY | APPROVED BY | |||
| Analyst – TU | Analyst – RU | HOD or designee – RU | QA – RU | |
| DESIGNATION |
|
|||
| SIGNATURE |
|
|||
| NAME |
|
|||
| DATE |
|
|||
Annexure-5- Method Transfer Waiver Certificate
- Objective:
- Certificate No.:
- Product Name:
- SOP No.:
- Receiving Location:
- Test(s), Test Methods (As STP No.) and Specifications
| Test(s) | Test Method (STP No.) | Specification No. | Specification Limits |
- Justification for Method Transfer Waiver, Evaluation, and Conclusion: (Tick the appropriate clause:)
-
- New method involves changes that do not substantially alter the ability to use the method at RU.
-
- Analytical procedure meant for transfer is the same or very similar to procedure already in use.
-
- The new dosage form possesses either a comparable composition or concentration of API relative to an existing product.
-
- Changes made in manufacturing process of API, which do not impact existing analytical procedure.
-
- Change of API source or recipe of drug product, which do not impact existing analytical procedure.
-
- The method is in use at RU for a long time, but AMT was not performed previously; provided there is no history of inconsistencies/failures.
-
- The personnel in charge of the development, validation or routine analysis of the product at the transferring unit are moved to the receiving unit.
-
- Any other (please specify)….
-
Elaborate the selected clause:
(An example for the condition in which new method involves changes that do not substantially alter the ability to use the method at RU is as below 🙂
-
- AMT successfully done earlier for ……………… (mention API/drug product name) as per STP No. …………… against the Protocol No. ……………… During the AMT, necessary training was imparted to the RU analyst(s).
-
- Since no change in analytical method(s) for………………. (mention name of the test(s) given in STP No. …………. (current STP No.),
-
- The AMT done for …………………….. per Protocol No. ……………………………is considered valid for the methods given in STP No. …….
- Therefore, the methods for …………………. (mention name of the test(s) given in STP No. ………………..is/are considered officially transferred.
| PREPARED BY TU |
APPROVED BY |
|||
| HOD or Designee – TU | HOD or Designee – RU | QA – RU | ||
| DESIGNATION |
|
|||
| SIGNATURE | ||||
| NAME | ||||
| DATE | ||||
Annexure-6- Analytical Method validation work flow
Annexure-7- Method Transfer Protocol Register
|
Sr No |
Product Name | Protocol No
(Product code / Version No) |
Name of receiving laboratory | Start Date | Completed date |
Remarks |
Annexure-8- Method Transfer Template Register
|
Sr. No. |
Product name | Protocol No
(Product code / Version No) |
Name of Transferring laboratory | Test Details | Template No |
Template approval date |
|
Issuance No |
Issued by/date | Issued to/date | Reason for Issuance | Start Date | Completed date |
Remarks |



Pingback: Tech Transfer Documents Handling Procedure - Guidelines - SOPs