 Standard Operating Procedure for Handling of Out of Calibration (OOC) for Laboratory Instrument and Equipment. The result which does not meet the pre-established acceptance criteria for the instrument calibration shall be termed as Out of Calibration (OOC).
Standard Operating Procedure for Handling of Out of Calibration (OOC) for Laboratory Instrument and Equipment. The result which does not meet the pre-established acceptance criteria for the instrument calibration shall be termed as Out of Calibration (OOC).
SOP for Handling of Out of Calibration (OOC) Results
1.0 PURPOSE
-
- The purpose of this Standard Operating Procedure (SOP) is to lay down the procedure for reporting and handling out of calibration (OOC) events for the laboratory instruments used in the Quality Control Laboratory.
2.0 SCOPE
-
- This SOP shall be implemented as such for Handling of Out of Calibration (OOC) events for the Laboratory Instruments used in quality control departments, at the pharmaceutical manufacturing plant.
3.0 RESPONSIBILITY
-
-
The Analyst of Quality Control Department (QC) shall be responsible for:
-
-
- To immediately inform the Head QC/ Section Head (GLP) or designee about OOC and shall not discard any solution preparation, glassware used and instrument settings until the evaluation of the OOC results.
-
- To participate in the investigation, participate in finding of root cause and to carry out the experimental analysis where applicable.
-
-
Section Head (GLP) or designee shall be responsible for :
-
-
- To carry out the investigation for OOC result as per approved effective SOP’s.
-
- To review historical data of laboratory investigation during the initial assessment to determine if Out of Calibration (OOC) has occurred previously, what were the corrective action (CA) and preventive action (PA) and the effectiveness of CA & PA.
Related: SOP for Operation and Calibration of UV Cabinet
-
- Interview with the analyst to find out the root cause for Out of Calibration (OOC) results.
-
- To review the Calibration Procedure (Protocol/ADS), and discuss with expert/service engineer to find the root cause for Out of Calibration (OOC) event.
-
- Initiation of the actions recommended in the Out of Calibration (OOC) investigation form.
-
- To derive CA & PA based on the assignable cause identified, perform impact assessment & risk assessment, and training.
-
-
Head QC or Designee shall be responsible for :
-
-
- To provide guidance for investigation.
-
- Review and conclude the Out of Calibration (OOC) Form.
-
- To initiate the actions recommended in the Out of Calibration (OOC) Form.
-
- Based on assignable cause determination of CA & PA and perform impact & Risk assessment and training.
-
- Periodic review of Out of Calibration (OOC) Trends and evaluation of the effectiveness of CA and PA derived based on OOC.
-
- To arrange for maintenance of the instrument.
-
-
Technical Staff of Quality Assurance Department (QA) shall be responsible for :
-
-
- Register the Out of Calibration (OOC) details in the “Laboratory OOC logbook” (Refer Attachment – 1).
-
- To issue the Out of Calibration (OOC) evaluation form (Refer Attachment – 2).
-
- Ensure that investigation shall be performed as per approved effective SOP’s.
-
- To review the OOC investigation/evaluation form.
-
- Based on the review, if required QA can revise the scope of CA & PA, Impact assessment, and Risk assessment.
-
- To ensure the execution of CA and PA recommended in the Out of Calibration (OOC) investigation form.
-
- To perform periodic trending (Quarterly) and review of Out of Calibration (OOC) investigation and evaluation of the effectiveness of CA & PA derived based on the OOC investigation trend.
-
- Archive the Out of Calibration (OOC) investigation report.
-
-
Head QA or designee shall be responsible for :
-
-
- To conclude and approve the Out of Calibration (OOC) investigation form.
-
- Ensure the execution of CA & PA recommended in the OOC investigation form.
Related: SOP for GC Column Receipt, Performance Check and Storage
-
- To guide for further investigation and/or action, wherever required.
-
- Intimation to the regulatory department and Customer wherever necessary.
4.0 PROCEDURE FOR HANDLING OG OUT OF CALIBRATION (OOC) :
-
- The purpose of the investigation is to identify the root cause of the OOC Event. The investigation shall be thorough, timely, unbiased, well documented, and scientifically sound.
-
- If Out of Calibration (OOC) results observed, the analyst shall not discard sample solution/stock solution/ instrument settings until the evaluation of Out of Calibration (OOC) results.
-
- QC analyst shall perform calibration of the instruments as per the SOP and complete the entries of raw data and observations in the calibration template / Protocol / Analytical worksheet.
Related: SOP for Disintegration Apparatus (DT)
-
- In case an instrument fails to meet any of the calibration criteria, QC analyst shall inform to Head QC or designee and affix a label / update the status for “Out of Calibration the respective instrument.
-
- Head QC shall intimate to Head QA or designee regarding the occurrence of OOC and ask for the OOC evaluation form.
-
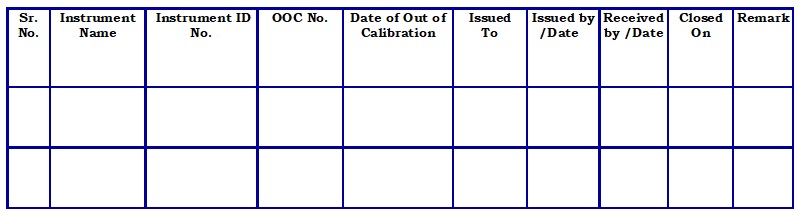
- The technical person of the QA department shall review the calibration template/report and then register details in “Out of calibration evaluation form issuance log” (Refer Attachment – 1) and issue an “Out of calibration evaluation form” (Refer Attachment -2) to Technical person of QC department.
-
-
“Out of Calibration Evaluation Form ” can be numbered as “OOC/AA/BBB”,
-
-
-
- Where,
-
-
-
- OOC stands for Out of calibration.
-
-
-
- AA stands for the last two digits of the current year ( for the year 2020, ‘AA’ shall be ‘20’).
-
-
-
- BBB stands for serial numbers starts from 001.
-
-
-
- e.g. First out of calibration of the instrument in the year, 2020 shall be labeled as “OOC/20/001”.
-
-
- While the issuance of the form, QA shall make necessary entries in the log for “Sr. No.”, “Instrument Name”, “Instrument ID/Code No.”, “OOC No.”, “Date of out of calibration”, “Issued to” “Issued by/Date” columns of the Out of calibration (OOC) evaluation form issuance log.
Related: Operation and Calibration of Analytical Balance
-
- After making entries in the register, QA shall make necessary entries in Out of calibration (OOC) evaluation form for, “Form No.”, “Issued To” and “Issued by/Date”, “Name of Instrument”, “Instrument ID No.”, “Reference SOP No.”, “Description of failure”, ” Calibration parameter”, “Result”, ” Acceptance criteria” “Calibrated by” and ” Date of calibration” columns.
-
- Section Head (GLP) or designee shall investigate the failure in calibration as per, but not limited to, the Preliminary investigation check-list given in the Out of calibration evaluation form (Refer Attachment –2) and record the observations as discussed with the QC analyst-1.
-
-
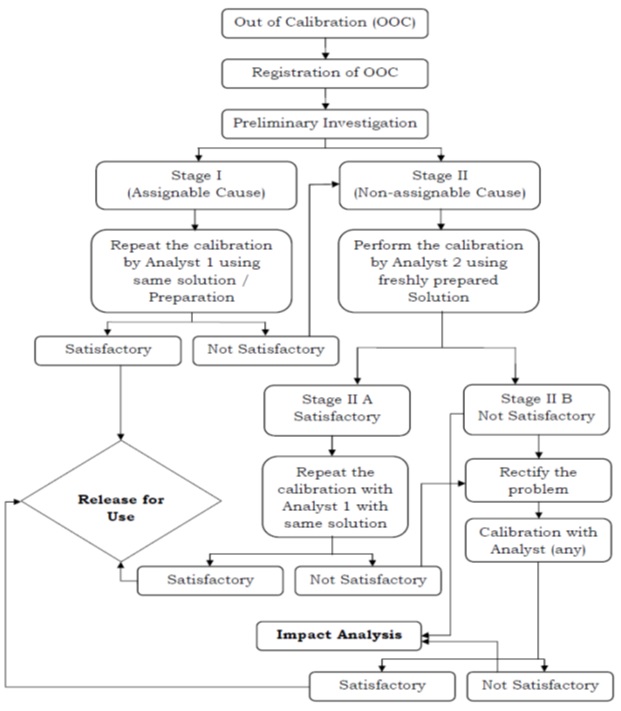
Stage I – Out of Calibration – OOC (If the cause for failure is assignable)
-
-
- Head QC or designee shall conclude it in the form and instruct the same analyst (Analyst -1) to repeat the calibration of the instrument with the same solution after rectifying the cause of the earlier failure.
-
- In case of the unavailability of the same analyst, Head QC can allocate the calibration to other QC analysts.
Related: SOP for Rounding off the Analytical Test Results
-
- QC analyst shall record the raw data and observations in issued calibration template/ADS / Protocol and intimate results to the Head QC or designee.
-
- Section Head (GLP) or designee shall enter the results in the “Result /Observations” column of the form and conclude.
-
-
Note:
-
-
- If failure is due to malfunction (The malfunction may be created due to the power outage, improper handling, breakage of any part which seen apparently, etc.) of the instrument then Head QC or designee shall conclude accordingly in the form and shall evaluate the impact with a consultation with head QA.
-
-
If Calibration found satisfactory
-
-
- Section Head (GLP) or designee shall release the instrument for use, after making necessary entries in the “Remark(s)” column of the form.
-
-
If Calibration found not satisfactory
-
-
- Section Head (GLP) or designee shall follow the procedure for the next stage as per flow chart,
-
-
Stage II (To verify analytical practices/errors of Analyst — 1)
-
-
- During preliminary investigation, if the assignable cause is not identified, then solution preparations need to be investigated.
-
- Section Head (GLP) or designee shall instruct another analyst (Analyst – 2) to perform the calibration of the instrument, with fresh calibration solutions, in presence of Section Head (GLP) or designee.
-
- Analyst — 2 shall record the raw data and observations in issued calibration template and intimate results to the Head QC or designee.
-
-
Stage II A (If Calibration found satisfactory)
-
-
- Section Head (GLP) or designee shall enter the results in the “Result /Observations” column of the form.
-
- Section Head QC or designee shall instruct Analyst — 1 to repeat the calibration with the same calibration solutions.
Related: Calibration of UV Spectrophotometer
-
- If calibration found satisfactory, Section Head (GLP) or designee shall enter the results in the “Result /Observations” column of the form and conclude in the form. and shall release the instrument for use, after making necessary entries in the “Remark(s)” column of the form.
-
- Head QC or designee shall re-train Analyst — 1 if required.
-
- If calibration found not satisfactory, Section Head (GLP) or designee shall make necessary entries in the “Remark(s)” column of the form and conclude.
-
- Head QC or designee shall intimately Instrument Engineer for rectification of the problem.
-
- Instrument Engineer shall fill the form in the “Instrument maintenance ” section of Attachment –2.
-
- Section Head GLP or designee shall hand over the form to QA for further evaluation.
-
- Head QA or designee shall evaluate the impact of the failure.
-
- After rectification of the problem, Section Head (GLP) or designee shall instruct Analyst- 2 to perform the calibration.
-
- If calibration found satisfactory, follow the procedure defined in stage 1.
-
- If Calibration found not satisfactory, proceed as per Stage II B.
-
-
Stage II B (If Calibration found not satisfactory)
-
-
- Follow the procedure as per stage 1.
-
- Note: If required additional page/s of OOC form can be reissued by mentioning respective remarks in the “Remarks” column of OOC Form issuance register against the same allocated Form No.
-
-
Impact Analysis
-
-
- QA shall evaluate the impact analysis (Through respective “Incidence” handling SOP) of the calibration failures from stage-II A and B and fill the form in Impact analysis. (Refer Attachment –2).
-
- Make a list of the samples analyzed between previous calibration and date of out of calibration occurrence.
-
-
Select the product for reanalysis based on the following criteria but not limited to,.
-
-
-
- The sample was released at the narrow margin to the specification.
-
-
-
- The last sample analyzed on the instrument.
-
-
-
- Based on the criticality of system suitability criteria of earlier released samples.
-
-
- QA shall make necessary entries in the ” Sample Requisition” column of the form and take authorization from Head — Quality Assurance.
-
- Arrange for analysis of the selected samples on a different instrument.
Related: HPLC Calibration- A Complete Guide
-
- Note: In case of non-availability of another instrument, analysis can be done on the same instrument after rectification of the problem and subsequent calibration, or any other laboratory (Contract Lab) where an equivalent instrument is available.
-
- Head QC or Designee shall-
-
-
- Conclude the investigation making a note in the “Conclusion of impact analysis” column of the form.
-
-
-
- Recommend corrective and/or preventive action(s) if any, and forward the completed form to Head QA for approval.
-
-
- Head QA shall-
-
-
- Evaluate the investigation and approve the form.
-
-
-
- Direct for further investigation if required.
-
-
-
- Decide the further course of action in case of sample(s) failure, by taking event/incidence, if required.
-
Related: Good Laboratory Practices for Workbench
-
- If the drug substances fail in impact analysis, then QA Head shall inform the regulators/customer whom the batch is supplied. (wherever required).
-
- Head QA or designee shall recommend and issue CAPA, if necessary to the concerned department for the monitoring of long term Corrective and/or Preventive action.
-
- Head QC shall ensure the completion of the recommended corrective and/or preventive action(s).
-
- After completion of the above, Section Head (GLP) shall submit the completed OOC Form to QA.
-
- QA shall mention “Closed” in the Status column of OOC and put Sign and Date in respective column and archive the OOC evaluation form.
-
- OOC investigation shall be completed within 30 calendar days from the date of reporting out of calibration (OOC) results.
-
-
Trending and Evaluation of Out of Calibration (OOC) :
-
-
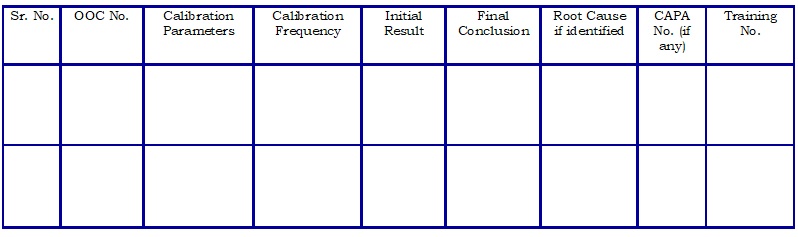
- Section Head GLP shall maintain the “Register for Trending and evaluation of OOC” (Refer Attachment – 3).
-
- Assess the OOC on a yearly basis and evaluate the Repetitive type of failures.
-
- If numbers of failures are more than two in a year shall be considered as repetitive type failures.
-
- Review the data based on a yearly basis and evaluate the repetitive type of failures as per following but not limited to,
-
-
- Repetitive OOC in a specific calibration parameter/analytical method.
-
-
-
- OOC (Repetitive) by the specific analyst.
-
-
-
- Repetitive OOC on a specific instrument.
-
Related: Analytical Solution Stability
-
- Conclude the repetitive failure(s) from data, based on which, training / Corrective and/or preventive action to be planned.
-
- Where the repetitive failure is significantly increased, a high level of investigation shall be planned to identify the root cause.
-
- A report shall be prepared for such a high level of investigation (or umbrella investigation) in support of the justification of repetitive failure and corrective and/or preventive action.
5.0 REFERENCES
-
- In-house
6.0 ABBREVIATIONS AND DEFINITIONS
-
- ADS: Analytical Data Sheet.
-
- OOC: Out of Calibration
-
- Definition of Terms :
-
- Assignable cause: A cause that has been identified as the reason to invalidate a test result. The assignable cause is a conclusion derived from direct or indirect evidence found during the investigation process, from the interpretation of analytical data or a combination of both.
-
- Out of calibration (OOC): The result which does not meet the pre-established acceptance criteria for the instrument calibration shall be termed as OOC.
7.0 ATTACHMENTS – OUT OF CALIBRATION (OOC) :
Flow Chart for Handling Out of Calibration event. (Attachment – 1)
Out of Calibration Evaluation form issuance log. (Attachment – 2)
Out of Calibration Evaluation form. (Attachment – 3)
| Form No. | : | Issued to | : | |||||
| Issued by/Date
(QA) |
:
|
Reference SOP No. | : | |||||
| Name of Instrument | : | Instrument ID No. | : | |||||
| Description of Failure :
|
||||||||
| Calibration parameter : | Result : | Acceptance criteria : | ||||||
| Calibrated by | Date of Calibration | |||||||
Preliminary Investigation
| Sr. No. | Parameters | Observation | Sign/Date |
| 1. | Status of calibration for other equipment / instrument(s) / devices used | ||
| 2. | Verification of calibration standards used
Primary standard : Physical appearance validity certificate Secondary standard : Physical appearance validity |
||
| 3. | Verification of dilution, calculation, weighing and readings. | ||
| 4. | Verification of glassware used | ||
| 5. | Verification of chromatograms/ spectrum’s/ other instrument printouts etc. |
| Sr. No. | Parameters | Observation | Sign/Date |
| 6. | Adequacy of system suitability checks | ||
| 7. | Instrument Malfunction | ||
| 8. | Check for adherence to the calibration method | ||
| 9. | Discussion with analyst for his/her understanding of the calibration methodology | ||
| 10. | Others:
|
| Remark(s) : (Summary of the investigation by the investigator (based on Raw data checking/discussion/previous experience). |
|
Conclusion: (Tick √ whichever applicable) Cause assignable c / Cause non-assignable c Assignable Cause details : |
| Stage -I
If found the assignable cause ( Under preliminary investigation) Repeat the calibration with the same solution after rectifying the cause by Analyst- 1( same analyst ) |
|
| Allotted by/date : | Allotted to/date : |
| Result : | Calibrated by/date : |
| Conclusion Of Stage I : (Tick √ whichever applicable) Calibration satisfactory c /Calibration not satisfactory c | |
| Remark(s) : | |
| Section Head (GLP) sign/ date : | |
| Stage -II
If Found non-assignable cause (Under preliminary investigation) Perform the calibration with fresh solution by Analyst- 2 (Different Analyst ) |
|||
| Allotted by/date : | Allotted to/date : | ||
| Result : | Calibrated by/ Date : | ||
| Conclusion Of Stage II : (Tick √ whichever applicable) Calibration satisfactory c /Calibration not satisfactory c | |||
| Remark(s) : | |||
| Section Head (GLP) sign/ date : | |||
Instrument maintenance |
|
| Intimation was given to the maintenance/instrument dept …………………………………………………… | Attended by/date: ………………….. |
| Maintenance detail(s) : | |
| Head QC or designee’s remark(s) : | |
| Head QC or designee’s sign/ date : | |
| Impact analysis (If fails in Stage II and Stage IIA, Stage IIB) | ||||||
| Sample requisition
· For drug substance/ intermediate/raw material re sampling follow the SOP of “sampling procedure”(SOP NO ._____________) . |
||||||
| Sr. No. | Name of product | B.No./ A.R. No. | Quantity required | Selection criteria | Sample to be withdrawn from | Remark |
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
|
|
||||||
| Requested by/date
(QC):
|
Approved by/date
( Head QA) |
|||||
Register for Trending and evaluation of OOC. (Attachment – 4)



Pingback: Agilent HPLC - Operation & Calibration SOP - Pharma Beginners
Pingback: FTIR-Operation and Calibration SOP - Pharma Beginners
Pingback: SOP - Corrective Action and Preventive Action (CAPA) - Pharma Beginners
Pingback: Melting Point Apparatus - Operation & Calibration - Guidelines - SOPs
Pingback: SOP for Instrument Calibration (Internal & Third Party) - Pharma Beginners