Standard Operating Procedure (SOP) for Handling of Market Complaint of Finished Pharmaceutical Drug Product. A market/consumer complaint is a notification that the product in commercial distribution-
-
- May be in violation of the laws or regulations administered by the FDA.
-
- May have caused illness, injury or death.
-
- Is alleged to have caused problems not covered by the above.
Guideline for Handling of Market Complaint
1.0 Purpose:
-
- To provide a guideline for handling market complaints of Drug Products.
2.0 Scope:
-
- This guideline is applicable to the handling of complaints of drug products at the formulation manufacturing locations of Pharmaceutical Industries.
3.0 Reference:
-
- References :
-
- 21 CFR, Part 314.80 and part 211.198l
4.0 Responsibility :
-
-
Corporate Quality shall be responsible for –
-
-
- Receive and log in to the market complaint in the market complaint register.
-
- Collect the adequate information required and the Complaint sample, if available, for complaint investigation.
-
- Forward the complaint to the concern location for the investigation.
-
- Receive Investigation Report or Preliminary Investigation Report from the location.
-
- Review, if required, Investigation Report or Preliminary Investigation Report received from the location.
-
- Send the investigation details to Complainant / Distributing locations / Distribution Department.
-
-
Subject Matter Expert shall be responsible for –
-
-
- Investigate the complaint.
-
- Prepare the investigation report.
-
- Get the Investigation Report reviewed and approved by concern persons.
-
-
QA Department (Manufacturing Location) shall be responsible for:
-
-
- Prepare location SOP based on this guideline.
-
- Log complaints in the market complaint register.
-
- Prepare PIR in case of quality-related market complaints where the investigation may take time.
-
- Prepare an action plan for investigation of the quality-related complaint and to get it approved.
-
- Coordinate market complaint investigation as per the action plan.
-
- Review the Investigation Report of the market complaint.
-
- Send a copy of the Investigation Report to CQ.
-
-
Quality Control Department shall be responsible for –
-
-
- Monitor the analysis of complaint sample and/or control sample as per the approved action plan.
-
- Provide analytical results or any other data with the conclusion.
-
-
Production Head (Manufacturing Head and Packing Head) shall be responsible for-
-
-
- Cooperate with QA in the process of investigation as per the action plan.
-
- Review the Investigation Report.
-
- Implement CAPA as defined in the investigation report.
-
- Head – Quality of manufacturing location shall be responsible for-
-
- Receive complaint from CQ or any other source and to handover to QA for investigation
-
- Review and approve the action plan
-
- Provide guidance for the investigation of the market complaint.
-
- Review and approve the Investigation Report.
-
- Head – Location of manufacturing location shall be responsible for-
-
- Review and approve the action plan
-
- Provide guidance for the investigation of the market complaint.
-
- Review and approve the Investigation Report with special reference to CAPA. .
-
- Facilitate resources for CAPA implementation.
-
-
Distributing location (for Loan License / Third Party Products) shall be responsible for-
-
-
- To file complaints, received for the products distributed by the distributing location and send it to the parent manufacturing company for investigation.
-
- To receive the Investigation Report from the parent manufacturing company and take further action as per it’s location SOP.
-
-
Distribution Department (Complaints related to Indian Market only) shall be responsible for –
-
-
- Arrange for replacement sample in case of the genuine market complaint as and when required with a copy to CQ.
-
- Prepare and send a reply to the customer/doctor as and when required with a copy to CQ.
5.0 Definition of terms & abbreviations (Market Complaint):
-
-
Market Complaint :
-
-
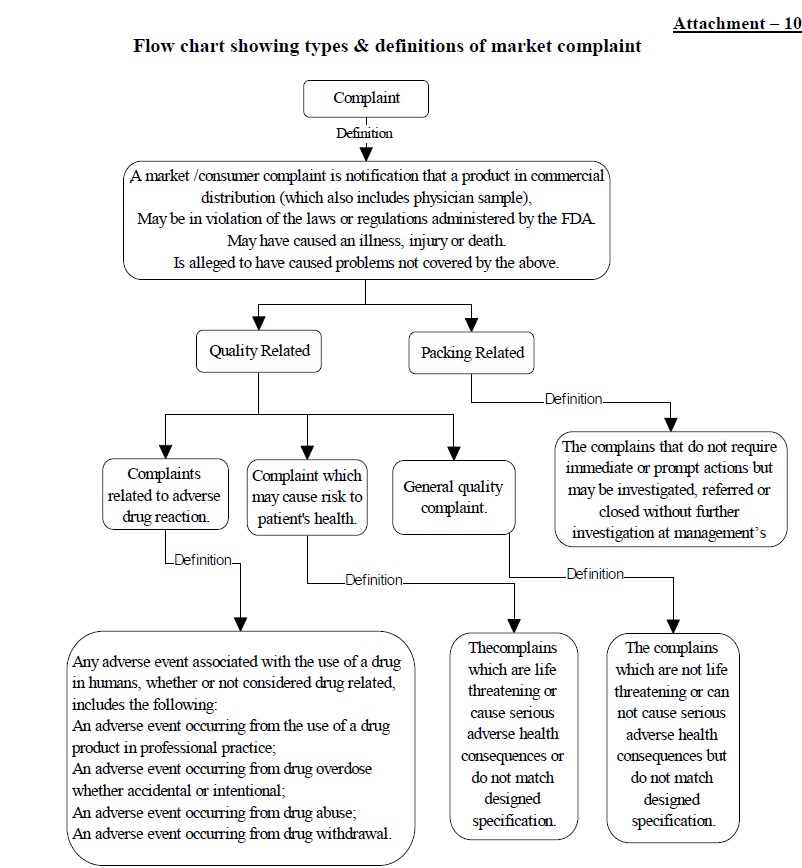
- A market/consumer complaint is the notification that a product in commercial distribution (which also includes physician sample),
-
-
-
- May be in violation of the laws or regulations administered by the FDA.
-
-
-
-
-
- May have caused illness, injury or death.
-
-
-
-
-
- Is alleged to have caused problems not covered by the above.
-
-
-
-
Adverse drug experience :
-
-
- Any adverse experience associated with the use of a drug in humans, whether or not considered drug-related, includes the following :
-
-
- Adverse experience occurring from the use of a drug product in professional practice;
-
-
-
- An adverse experience occurring from drug overdose whether accidental or intentional;
-
-
-
- Adverse experience occurring from drug abuse;
-
-
-
- An adverse experience occurring from drug withdrawal and
-
-
-
- Any failure of expected pharmacological action.
-
-
-
Life-threatening adverse drug experience :
-
-
- Any adverse drug experience that places the patient, in the view of the initial reporter, at immediate risk of death from the adverse drug experience as it occurred, i.e. it does not include an adverse drug experience that, had it occurred in a more severe form, might have caused death.
-
-
Serious adverse drug experience :
-
-
- Any adverse drug experience occurring at any dose that results in any of the following outcomes :
-
- Death, a life-threatening adverse drug experience, in-patient hospitalization.
OR
-
- Prolongation of existing hospitalization, a persistent or significant disability/incapacity, or a congenital anomaly/birth defect.
-
- Disability: A substantial disruption of a person’s ability to conduct normal life functions.
-
-
Unexpected adverse drug experience :
-
-
- Any adverse drug experience that is not listed in the current labeling for the drug product.
-
- This includes experiences that may be symptomatically and path physiologically related to an event listed in the labeling but differs from the event because of greater severity or specificity.
-
- “Unexpected”: As used in this definition, refers to an adverse drug experience that has not been observed previously (i.e. not included in the labeling) rather than from the perspective of such experience not being anticipated from the pharmacological properties of the pharmaceutical product.
-
- Factory Management: Plant Head and Quality Head together are termed as Factory Management.
-
- Subject Matter Experts: Individual or a group of individuals who has a better understanding of the matter either through qualification or training or experience or combination of above.
-
-
Abbreviations :
-
-
- ADE: Adverse Drug Event.
-
- C and F Agent: Carrying and Forwarding Agent.
-
- CAPA: Corrective Action & Preventive Action
-
- CRA: Corporate Regulatory Affairs Department
-
- FAR: Field Alert Report
-
- FDD: Formulation Development Department.
-
- NDA: New Drug Application.
-
- OOS: Out of Specification
-
- OOT: Out of Trends
-
- PIR: Preliminary Investigation report.
- RA: Regulatory Affairs
-
- SME: Subject Matter Experts
6.0 Procedure for the handling of Market Complaint :
-
-
Source of complaint :
-
-
- A complaint may be received from Consumers, Healthcare professionals, government / regulatory agencies, trade sources, C and F agents or from any other source, in any form (written or verbal, post, fax, email or through the website).
-
- The Market Complaint can be received by Marketing personnel, Head Office, CQ or Manufacturing location.
-
- All complaints, for the products manufactured in India, received at the location, by any other employee of the company or any other office, shall be forwarded to CQ for registration.
-
- However, the location can assign location complaint numbers and start an investigation without waiting for the CQ registration number.
-
- On receipt of the CQ registration number, the same shall be updated in the register.
-
- Market Complaints about other (overseas) locations shall be received and registered by respective manufacturing locations. Only critical complaints shall be informed to CQ.
-
-
Receipt, Registration, and Classification of Market Complaint:
-
-
- On receipt of the market complaint, CQ shall classify these complaints into Quality complaints (Quality of product or complaint related to primary packing of product) and shortage complaints (Complaints of shortages or excess related secondary packing or tertiary packing) and register them in register as per attachment – 1 and attachment – 2 This register shall be maintained in soft file.
-
- For complaints from Indian Market, in order to understand the nature of the complaint, efforts shall be made by CQ to discuss and understand the nature of complaints by contacting Doctors or Nursing Staff directly or through field staff in the marketing department.
-
- For complaints from other locations, similar efforts shall be made through appropriate channels.
-
- This can be by QA in the US, Canada, Mexico, etc. or by QP in EU or through other personnel as appropriate in other countries.
-
- For the complaints from the US market, related to products manufactured at the Indian Manufacturing location, CQ in consultation with Corporate Regulatory Affairs shall decide on the need to file a field Alert Report.
-
- For products manufactured at other (overseas) locations such decision locations shall be taken by location QA in consultation with location RA.
-
- Similar reporting to other regulatory agencies, if applicable, shall be done.
-
-
CQ shall register quality and packing complaints in CQ Quality Complaint Register (attachment – 1) and assign a CQ complaint number as per MC-YY-ZZZ,
-
-
-
- Where,
-
-
-
- MC stands for Quality / Packing Market Complaint.
-
-
-
- YY stands for the last two digit of the year. 20 for 2020 and 21 for 2021 and so on
-
-
-
- ZZZ stands for Serial number starting from 001 for each calendar year.
-
-
-
- First complaint for the year 2011 shall be numbered as MC-20-001 and for the year 2021 shall be numbered as MC-21-001, and so on.
-
-
-
CQ shall register shortage/excess complaints in CQ Shortage Complaint Register (attachment – 2) and assign a CQ complaint number as per MCS-YY-ZZZ,
-
-
-
- Where,
-
-
-
- MCS stands for Market Complaint related to Shortages / Excess
-
-
-
- YY stands for last two digit of the year. 20 for 2020 and 21 for 2021 and so on
-
-
-
- ZZZ stands for Serial number starting from 001 for each calendar year.
-
-
- Count related complaints for tablets / capsules packed in bottles, Missing tablet/capsule in blister/strip, missing vial or ampoule in a combi pack, empty show box, shall be treated as packing related complaints and not as a shortage or excess complaints.
-
- Registration of complaint shall be completed within 2 working days.
-
- If complaints are already classified as ADE or non-ADE, all ADE complaints shall be forwarded to Drug Safety Department for records and PSUR purpose as applicable.
-
- If at the time of receipt complaints are not classified as ADE or non-ADE complaints then all quality-related complaints shall be evaluated for ADE or non-ADE complaints. Drug Safety Department shall be consulted if required.
-
- If any complaint is classified as ADE complaint, it shall be forwarded to the Drug Safety Department also.
-
- All quality complaints shall be further sub-classified into different categories as per Attachment – 3 and Attachment – 10.
-
- Complaints shall be forwarded to Quality Head or Designee at the respective manufacturing location.
-
- Location QA shall log these complaints into location complaint register (Attachment – 4) within 1 working day and shall allot a unique location complaint number.
-
- All complaints shall be logged in a common register.
-
- If applicable PIR shall be prepared as per the above procedure.
-
- Subject Matter expert shall investigate the complaint.
-
-
Investigation of the Market Complaint :
-
-
- Preliminary investigation :
-
- In the case of Quality Complaint where there is a serious risk to the patient’s health PIR shall be prepared within 2 working days.
-
- Quality Head shall decide on this. If required opinion from Medical Expert / Department can be obtained.
-
- Preliminary investigation for complaints classified as ADE complaint (other than those covered under above), shall be done within five working days from the date of receipt of the complaint.
-
- A preliminary investigation shall evaluate any abnormality during batch manufacturing which might be prima fascia responsible for such complaints.
-
-
In the case of ADE complaint, product literature shall be evaluated for :
-
-
-
- Listed ADEs, contraindications, dosing, route of administration, warning, any other precaution, etc.
-
-
-
- The information regarding the use of suspected drug products in combination with other drug products.
-
-
-
- The patient history.
-
-
- Based on the batch record and product literature evaluation a Preliminary Investigation Report (PIR) shall be prepared as per attachment – 6.
-
- The location shall forward the PIR to Corporate Quality Department for forwarding to the complainant, if found satisfactory, if CQ has any queries or suggestions for improvement, PIR shall be returned back to the location for updation.
-
- Updated PIR shall be forwarded to CQ Department for onward submission.
-
- Location QA shall file the PIR. On completion of the investigation, the PIR shall be attached with the market complaint Investigation Report.
-
-
Detailed investigation of Market Complaint:
-
-
- SME shall Investigate the complaint in detail to identify the root cause of the complaint.
-
- QA shall co-ordinate with CQ and try to get as much information as possible from the complainant and / or Healthcare professional.
-
- Such efforts should be documented and enclosed with the Final Investigation Report.
-
- All efforts shall be made to secure the complaint sample for effective investigation.
-
- Batch manufacturing and packing records and all other related records shall be reviewed to evaluate the possible root cause of the complaint.
-
- If the batch number is unknown, Batches manufactured within the last 6 months shall be evaluated with special reference to events / OOS or OOT that could be attributed to the root cause of the complaint.
-
- Depending on the nature of the market complaint, QC analytical data shall also be reviewed. If required stability data and trend data shall also be reviewed.
-
-
Control samples and Complaint samples, if available, shall be inspected.
-
-
- Control samples and complaint samples (if available) shall be analyzed for relevant parameters to establish the acceptability of the product.
-
- However, if the complaint samples are received in suspicious conditions, justification for not analyzing the complaint samples shall be documented.
-
- If required, the hypothesis shall be proved by experimentation.
-
- Based on the root cause identified during the investigation, impact assessment on the complaint batch and other batches of same product or different product manufactured on the same line shall be evaluated.
-
- If the market complaint is confirmed, the risk-based decision on the marketed batches shall be evaluated and if required further actions such as FAR (for US marketed products only), or similar reporting as applicable, Recall, etc. shall be taken.
-
-
Market complaint investigation shall be completed in 30 days from the receipt of the complaint.
-
-
- If in these 30 days complaint samples are not received or complaint samples are received but not analyzed, complaint investigation except for analysis of complaint samples shall be concluded within 30 days.
-
- Addendum report, including analytical results and additional investigation if carried out based on the analytical results of complaint/control samples, shall be prepared within 15 days from receipt of complaint samples except for tests where test period is more than 3 days, such as sterility test, antimicrobial efficacy test, Microbial Limit test or Biological Assays, shall be completed within 7 days after receipt of analysis results.
-
- Extension to all these timelines can be given by the Quality head or his designee subjected to appropriate justification for extension (Refer attachment – 7).
-
- Extension notes shall bear unique numbered.
-
- A log shall be maintained in the register as per attachment – 9.
-
- The extension request note shall be attached to the Investigation Report.
-
- The market complaint report shall be compiled as per the format (Refer Attachment – 8).
-
- Based on the root cause identified during the investigation, impact assessment shall be done on the batch(es) of the same product or different products handled on the same manufacturing line.
-
- Remaining quantities of complaint samples after completion of the investigation, shall be retained in the control sample room till one year after expiry of the product.
-
-
Market Complaint samples received without a specific batch number shall be retained for 1 year from the date of receipt of the complaint sample.
-
-
- If the complaint sample cannot be retained because of the nature of the product or any other reason, the justification for not retaining complaint samples shall be documented in the investigation report and a photograph of complaint samples shall be kept in record.
-
- QA shall send a copy of the Investigation Report to CQ along with necessary attachments.
-
- CQ may review the Investigation Report and ask for further investigation details if required.
-
- CQ shall send investigation details to the Complainant / Distributing location.
-
- After completion of the investigation, if required, location QA shall initiate CAPA as per SOP for Corrective and Preventive Action.
-
- If required, Formulation Development Department or Packing Development Department shall be involved for adequate CAPA.
-
- The risk-based decisions shall be taken for the batch(es), which are impacted.
-
-
Handling of Repetitive ADE complaints :
-
-
- If ADE market complaint of similar nature is reported by more than one complainant at a time, CQ shall instruct location to prioritize the investigation and based on investigation outcome further decision on batch shall be taken.
-
- The location shall investigate the complaint as per the investigation procedure explained onwards and sent Investigation Report to CQ.
-
- If the ADE is not listed in Product literature then-current Product Literature of Reference (Innovator) drug shall be reviewed to ensure that there are no updates in the innovator’s literature.
-
- The matter shall be discussed with the Medical department and CRA department to evaluate any need for revision in product literature.
-
-
Reply to Market Complainant :
-
-
- If required, the Distribution Department shall send the initial response with/without replacement sample to the complainer and send a copy to CQ.
-
- Subsequently, CQ shall send a copy of the initial response to the concern location for record purposes.
-
- After receipt of the Investigation Report, CQ shall send either investigation details or reply for the complaint to the complainant or concern department with a copy to the concerned location for record purpose.
-
- For all export-related complaints, responses sent to complainer/business partner shall be forwarded to location also for their record purpose.
-
- The location shall file this response along with the investigation report.
-
-
Closure of Quality Complaints
-
-
- Closure of Complaints where CAPA is defined.
-
-
- Such reports shall be closed in 2 stages. Level – I closure shall be after approval of the investigation report by Factory Management. Level – II closure of such a report shall be done after the implementation of CAPA.
-
-
- Closure of Complaints where no CAPA is defined or CAPA defined is already completed :
-
-
- Market Complaint shall be closed for both level – I and Level – II simultaneously (i.e. with approval of Investigation report by the factory Management).
-
-
-
- NOTE: If the market complaint is closed and on a later date any communication is received from the complainant giving additional information or providing clarity about information provided earlier and that new information/clarification may have an impact on investigation then the complaint file shall be re-opened and an addendum Investigation Report shall be prepared.
-
-
-
Handling of Secondary or tertiary Packing Related complaints (Shortage / Excess Receipt or Improper packing) :
-
-
- NOTE: Complaints related to primary packing (such as Count variation in the bottle, empty pocket, missing component, missing label or batch details on the unit pack, missing applicator, empty vial/ampoule, leakage in vial/ampoule/bottle, Damaged primary packings such as bottle, ampoule or vial, etc.) shall be classified as Quality complaints. Refer attachment – 3.
-
- These are the complaints related to shortages or excess quantities of product in secondary packing (Less or excess vials/bottles/strips/blisters etc. in show box or Shipper boxes, Less or the excess number of show boxes in a shipper box, Damaged Outer or Inner Shippers, Missing labels on outer or inner shipper but proper labeling on the unit pack, missing leaflet/product literature (where applicable), the difference in weight mentioned on shipper and actual weight of the shipper on receipt, etc.).
-
- Location QA shall forward the complaints related to Shortage/excess to Packing Department Head. Department Head in consultation with quality Head shall identify the SME for investigation.
-
-
SME shall investigate the complaint to identify the root cause of the complaint.
-
-
- Based on the root cause identified during the investigation, impact assessment on the complaint batch and other batches of the same product or different product manufactured on the same line shall be evaluated.
-
- If the market complaint investigation indicates any CAPA then the risk-based decisions on the marketed batches shall be evaluated and if required further actions such as FAR (for the US marketed products only), or similar reporting to the regulatory agency as applicable, Recall, etc. shall be evaluated.
-
- Complete the Market complaint investigation in 30 days from the receipt of the market complaint.
-
- If market complaint investigation is not completed within 30 days an extension through extension request note (refer attachment – 7), justifying the delay in the investigation shall be submitted to Quality Head.
-
- Attache the extension request note the Investigation Report.
-
- Compile the market complaint report as per the format. (refer Attachment – 8).
-
- QA shall send a copy of the Investigation Report to CQ along with necessary attachments.
-
- CQ may review the Investigation Report and ask for further investigation detail if required
-
- CQ shall send Investigation Report to the appropriate Department / Complainant / Distributing location.
-
-
Closure of Shortage / Excess Related Market Complaints
-
-
- All shortage / Excess related complaints where no CAPA has been defined shall be treated as closed once Investigation Report is signed by the factory Management.
-
- Complaint Investigation Reports in which CAPA has been defined shall be treated as closed for level – I after the signature of Factory Management. Level – II closure of such complaints shall be done after the implementation of CAPA.
-
-
Market Complaints related to Contract Manufacturing / Third Party Manufactured products :
-
-
- Complaints related to Contract Manufacturing / Third Party Products shall be received and registered as per procedure defined in point no. 6.2.1 to 6.2.6
-
- Complaints shall be forwarded to Third-party coordinator.
-
- The third-Party coordinator shall forward the market complaint to the respective manufacturing location for investigation.
-
- Market complaint investigation shall be completed as per the timelines defined in the technical agreement.
-
- The third-Party coordinator shall coordinate with the third party for the Investigation Report and shall forward the report to CQ.
-
- CQ may review the Investigation Report and ask for further investigation details if required
-
- CQ shall send Investigation Report to the concern Department / Complainant / Distributing location.
-
-
Trending of Market Complaints :
-
-
- Trends for Quality and packing related market complaints with reference to the product, nature of complaints, open and closed complaint, the time required for closure of complaint, CAPA Status, and its effectiveness shall be prepared on Half-yearly basis by location QA.
-
- Trends for shortages related market complaints with reference to the product, nature of complaints, Zone, CNF agent, open and closed complaint, time required for closure of complaint, CAPA Status, and its effectiveness shall be prepared on Half-yearly basis by location QA.
-
- Based on trending, if required, Umbrella investigation report shall be prepared.
-
- The decision for Umbrella investigation shall be taken by QA Head in consultation with Quality Head.
3.0 Attachments, and Annexures :
Attachments
Attachment – 1: Format of CQ – Quality Market Complaint Register.
Prepare the logbook by incorporating the following table contents…
-
- Complaint No.
-
- Date of complaint received
-
- Reference No.
-
- Product
-
- Batch No.
-
- Location
-
- Complaint details
-
- Nature of complaint
-
- Market
-
- Complaint received from
-
- Date of investigation completion
-
- Level – I closing
-
- Date on which Investigation Report sent to CQ
-
- Date on which Investigation Report sent to Complainant / Distributor
-
- Recommended Corrective actions ensured on
-
- Level – II closing
-
- Remarks
Attachment – 2: Format of CQ – Shortage market complaint register.
Prepare the logbook by incorporating the following table contents…
-
- Complaint No.
-
- Date of complaint received
-
- Product
-
- Batch No.
-
- Location
-
- Complaint details
-
- Complaint received from
-
- Market
-
- Date of investigation completion
-
- Level – I closing
-
- Date on which Investigation Report sent to CQ
-
- Date on which Investigation Report sent to Complainant / Distributor
-
- Recommended Corrective actions ensured on
-
- Level – II closing
-
- Remarks
Attachment – 3: Market complaint code classification.
| A | Quality |
| Q1 | Failing specification / Poor quality / Adulterated / Poor efficacy / Ineffectiveness. |
| Q2 | Change in Color / Physical form (caking or lump formation) / odor/ Turbidity / precipitation. |
| Q3 | Broken / Defective / Melted tablet / capsule. |
| Q4 | Suspended matter / Foreign matter / Microbial contamination. |
| Q5 | Unexpected results / Reaction / Adverse effect. |
| Q6 | PFS Leaking or actuated automatically. |
| B | Packing |
| P1 | Improper/ Missing overprinting on the primary pack. |
| P2 | Damaged strips / bottle / vial / Empty pocket in strip. |
| P3 | Defective dropper / Pumps / Rubber stopper / Seal / Applicator. |
| P4 | The missing label on the primary pack. |
| P5 | Empty bottle / vial / ampoules / Leakage / Volume variation / less volume / missing component in combipack. |
| P6 | Improper sealing. |
| P7 | Mix-up: Label / Show box / Foil. |
| P8 | The defective Autoinjector (non-operative or exploded) / Device not working. |
| P9 | Lessor excess count of tablets/capsules in the bottle. |
Attachment – 4: Format of location market complaint register.
Prepare the logbook by incorporating the following table contents…
-
- Complaint No
-
- Date of complaint received
-
- CQ Complaint No.
-
- Reference complaint no.
-
- Product
-
- Batch No
-
- Dosage form
-
- Complaint details
-
- Nature of complaint
-
- Complaint received from
-
- Market
-
- Zone
-
- Date of investigation completion
-
- CAPA required / Not required
-
- Level – I closing (by / date)
-
- Date on which Investigation Report sent to CQ
-
- Recommended CAPA
-
- Level – II closing (by / date)
-
- Remarks.
Note: Zone shall be applicable only for Domestic Market complaints
Attachment – 5: Example of market complaint with the guideline for investigation.
Examples of Market Complaint with Guideline for Investigation
Note: This attachment shows only a few examples of market complaint with the suggested investigation. This attachment shall be edited based on the type of formulation (sterile/non-sterile) manufactured at the location and also based on the type and trend of the complaint received at the location.
1 Ineffectiveness / poor quality / Inadequate response of the drug.
-
- History of the product.
-
- Physical inspection of complaint and control sample.
-
- Review of batch document for,
-
- Active RM calculation.
-
- added of active and inactive RM (MRO and Coupons) against bill of material.
-
- Source of material.
-
- Dispensing precautions: e.g. API dispensing and storage in the black/ light resistant bag or container.
-
- Processing precautions: e.g. dissolved oxygen, low light, nitrogen flushing or any other.
-
- Processing parameters.
-
- In-process checks by production and QA.
-
- Daily quality observation record.
-
- Any deviation, which has direct or indirect impact on product quality.
-
- In-process quality control data.
-
- Review of FP analytical report and trend.
-
- Review of stability data.
-
- Complaint and control sample analysis for,
-
- Volume variation,
-
- Content uniformity.
-
- Moisture content.
-
- Storage condition.
2 Less content in capsules / vial/ ampoules / bottle.
-
- Physical inspection of complaint and control sample,
-
- For minor crack.
-
- Improper sealing.
-
- Condition of container label and/or show box to eliminate the possibility of leakage.
-
- Review of batch manufacturing record for,
-
- Active RM calculation
-
- added of active and inactive RM (MRO and Coupons) against bill of material.
-
- In-process checks by production and QA
-
- Visual inspection record.
-
- Leak test record.
-
- Yield and reconciliation of the batch.
-
- In-process and FP quality control data.
-
- Trend of yield.
-
- Sequential log of filling or compression or capsule filling machine for the breakdown.
-
- Daily quality observation record.
-
- Market Complaint and control sample analysis for,
-
- Content uniformity.
-
- Volume variation.
3 Bulging of strip pockets.
-
- History of the product.
-
- Physical inspection of control and complaint sample.
-
- Improper storage condition.
-
- Review of stability data.
-
- Review of Packing material Quality.
-
- Analysis of complaint sample for,
4 Presence of foreign matter (Living / non-living).
-
- History of the product.
-
- Physical inspection of complaint and control sample for,
-
- Minor crack
-
- Improper sealing.
-
- Daily quality observation record.
-
- pH trend (to correlate with the leaching from the container).
-
- Physical inspection of RM / PM of particular AR No. used for manufacturing of the batch.
-
- Review of batch manufacturing record for,
-
- Use of pretreated ampoules (e.g. acid-treated amps).
-
- Empty primary Pkg. material washing and sterilization in the record.
-
- Cleaning record of manufacturing, filtration and filling equipment, and area.
-
- Sterilization record of filtration and filling equipment.
-
- Filter integrity test results (Pre and post-filtration)
-
- Leak test record.
-
- Terminal sterilization record.
-
- Sequential log of washing machine.
-
- Environmental monitoring data.
-
- Quality/compatibility of closure.
-
- Microbiological analysis of complaint sample.
-
- Training record of visual inspectors.
5 Adverse reactions
-
- Review of complaint history
-
- History of the patient.
-
- Review of the package insert.
-
- Microbiological analysis of complaint sample.
-
- Pharmacology of the API and related formulations.
-
- Analysis of complaint/control samples for :
-
- Assay / Dissolution
-
- Related Substances.
6 Discoloration of solution or tablet / Capsule
-
- History of the product.
-
- Physical inspection of complaint and control sample for,
-
- Minor crack.
-
- Improper sealing.
-
- Review of batch manufacturing record for,
-
- Special precautions required during processing e.g. dissolved oxygen, low light, nitrogen flushing or any other.
-
- Cleaning record of manufacturing, filtration and filling equipments and area.
-
- Leak test record.
-
- Terminal sterilization record.
-
- Daily quality observation record.
-
- Recovery procedure.
-
- In-process checks by production and QA during manufacturing and packing.
-
- Analysis of control and / or complaint sample for,
-
- Stability data.
-
- Storage condition.
7 Damaged / broken / leaking capsule.
-
- Physical inspection of complaint and control sample.
-
- Review of batch manufacturing record for,
-
- Visual inspection record and humidity conditions.
-
- Cap filling machine setting parameters.
-
- In-process checks during manufacturing and packing by QA and production.
-
- Vendor of the empty capsule.
-
- Sequential log of capsule filling machine for the breakdown.
-
- Training of the visual checkers.
-
- Compatibility study of empty hard gelatin cap with excipients.
-
- Monitoring of defoiling and repacking activity.
8 Broken Tablet.
-
- History of the product.
-
- Physical inspection of complaint and control sample.
-
- Review of batch manufacturing record for,
-
- In-process checks by production and QA during manufacturing and packing.
-
- Visual inspection record.
-
- Review of the trend of processing, in-process and FP parameters.
-
- Daily quality observation record.
-
- Handling of the bulk product.
-
- Training record of the visual checkers and strip packing machine operators.
-
- Analysis of control and/or market complaint sample for monitoring of de-foiling and repacking activity.
9 Melt back (of lyophilized cake).
-
- History of the product.
-
- Physical inspection of control and complaint sample.
-
- Review of batch document for,
-
- Filling in-process checks by production and QA
-
- Lyophilization menu.
-
- Visual inspection record.
-
- Hold time at different stages.
-
- and humidity conditions at different stages.
-
- Review of trend of processing, in-process and FP parameters.
-
- Daily quality observation record.
-
- Review of stability data.
-
- Analysis of complaint and/or control sample for,
-
- Moisture content
-
- Degradation
-
- Training record of visual inspectors.
10 Product or batch Mix up
-
- Physical inspection of control and complaint sample for physical appearance of primary pkg. the material of two products under question.
-
- System followed to ensure proper segregation products at different stages.
-
- Sequential log of machine at every stage to know the previous or next product taken on the same machine and to ensure absence of same /similar product in the surrounding area.
-
- Other products packed on the same day on the nearby labeling machine or packing line of the product under question.
-
- Review of batch manufacturing record for,
-
- Machine and line clearance record at different stages.
-
- Reconciliation of packaging materials.
- Reconciliation of bulk and FP.
-
- Analysis of control and complaint sample (if available) for,
-
- Identification test of two products under question.
-
- Wrong labeling/packing.
-
- Daily quality observation record.
-
- Monitoring of de-foiling and repacking activity.
-
- Training record of packers.
-
- Repacking if done at any C and F location.
11 Poor quality of cap (dropper), Applicator
-
- History of the product.
-
- Physical inspection of control and/or complaint sample.
-
- Vendor of pkg. (cap or dropper) material.
-
- Compatibility study.
-
- Review of stability data.
12 Fake product.
-
- History of the product.
-
- Comparison of complaint sample with control sample for the appearance of strip/ label (font size of letters, printed text matter, size of the pocket, the gap between the two pockets, knurling pattern, the logo of the company, movement of tab or cap in the pocket, etc).
-
- Market complaint sample with control sample for appearance of tablet or capsule (size or dimensions, color, imprint, embossing, edge type, etc).
-
- Comparison of primary packaging material (Vial / ampoule) for shape and size, amp. sealing height, type of seal, logo on the seal, color of the seal, type of rubber stopper, etc.
-
- Analysis of control and complaint sample (if available).
13 Empty primary container (Vial / ampoule / bottle / pocket of strip or blister)
-
- Physical Inspection of control and complaint sample (if available).
-
- Sequential log of filling or striping or blistering machine for
-
- Review of batch document for,
-
- In-process checks by production and QA during filling
-
- Leak test record.
-
- Visual inspection record.
-
- In-process checks by production and QA during packing (e.g. on line compressed air flow or any other system followed to remove empty plastic container or empty pocket in strip or blister).
-
- Yield and reconciliation of the batch and comparison with trend.
-
- Balance performance and calibration check record.
-
- Weight variation record of packed show boxes and/or shippers.
-
- Proper segregation of packed and empty boxes.
-
- Daily quality observation report.
-
- Training record of the visual inspectors.
-
- Vendor of the primary container (as the cause of empty container may be hairline cracks due to weak MOC of the container).
14 Receipt of product in different show box / having different label
-
- Complaint sample observation.
-
- Physical inspection of control sample.
-
- Previous and next product packed on the same machine.
-
- Appearance of packing material of two products under question.
-
- Review of batch document for,
-
- Line clearance (by packing and QA) record.
-
- Reconciliation of packing material.
-
- Machine and line clearance record.
-
- In-process checks by packing and QA.
-
- Daily quality observation record.
-
- Storage of packing material in the store and in pkg. Dept.
-
- Procedure to be followed for the leftover pkg. The material after completion of packing.
-
- Monitoring of de-labeling and relabeling/ repacking activity.
-
- Inspection of the remaining stock of PM of the products under question.
-
- PM vendor audit.
-
- Training of packers.
-
- Repacking if done at any C and F location.
-
Count variation of tablets and capsules in bottles
-
- Physical Inspection of control and complaint sample (if available).
-
- Sequential log of filling machine for the breakdown.
-
- Review of batch document for,
-
- Startup clearance.
-
- In-process checks by production and QA during filling
-
- Leak test record.
-
- Visual inspection record.
-
- Challenge tests/performance check for the sensors on line.
-
- In-process checks by production and QA during packing (e.g. on line compressed airflow or any other system followed to remove such container).
-
- Yield and reconciliation of the batch and comparison with the trend.
-
- Balance performance and calibration check record.
-
- Weight variation record of packed show boxes and / or shippers.
- Proper segregation of packed and empty boxes.
-
- Daily quality observation report.
-
- Training record of the visual inspectors.
-
Non-working Devices (Auto-injector pens, Prefilled syringes or devices for inhalation)
-
- Physical Inspection of control and complaint sample (if available).
-
- Sequential log of filling / assembling machine for the breakdown.
-
- Review of batch document for,
-
- Startup clearance.
-
- In-process checks by production and QA during filling
-
- Leak test record.
-
- Visual inspection record.
-
- Challenge tests/performance check for the sensors on line.
-
- In-process checks by production and QA.
-
- Yield and reconciliation of the batch and comparison with trend.
-
- Performance check record during assembling / filling.
-
- Daily quality observation report.
-
- Training record of the visual inspectors.
-
Missing Label / Missing batch details
-
- Complaint sample observation.
-
- Physical inspection of control sample.
-
- Review of packing materials used for packing of the batch.
-
- Review of batch document for,
-
- Line clearance (by packing and QA) record.
-
- Reconciliation of packing material.
-
- Machine and line clearance record.
-
- In-process checks by packing and QA.
-
- Performance check for sensors on line.
-
- Daily quality observation record.
-
- Storage of packing material in the store and in pkg. Dept.
-
- Procedure to be followed for the leftover pkg. The material after completion of packing.
-
- Monitoring of de-labeling and relabeling/ repacking activity.
-
- Inspection of the remaining stock of PM of the products under question.
-
- PM vendor audit.
-
- Training of packers.
-
- Repacking if done at any C and F location.
Note: Processing parameters include:
-
- Quality of input material.
-
- Source of input material (vendor).
-
- Quality of purified water and water for injection.
-
- Nitrogen flushing.
-
- Mesh size of the sifter, direction of knife.
-
- Mixing speed and time.
-
- Speed of mill and screen size used during milling.
-
- Type (MOC) of the filter used.
-
- Drying inlet and outlet temp. time, endpoint (LOD).
-
- Lubrication time, rpm of blender.
-
- Speed of compression machine.
-
- Dissolved oxygen and Humidity conditions at different stages.
-
- Ampoule sealing height.
-
- Sterilization cycle of empty primary containers.
-
- Porosity of filter used.
-
- Granulation time and qty. of binder consumed.
-
- Terminal sterilization cycle.
-
- Pre and post integrity test results of the filter.
-
- Leak test cycle.
-
- Strip packing machine speed of sealing roller.
-
- Hold time at different stages.
-
- Lyophilization cycle.
-
- Any special precautions to be taken like manufacturing under low light.
Attachment – 6: Format of Preliminary Investigation Report.
To :______________________________________________
From :____________________________________________
Date :________________
Subject : (Mention Reference location, CQ and third party if available, complaint numbers).
Product :___________________________________________
Batch No. :_____________
Manufacturing Date :_____________
Expiry Date___________________
Pack style and NDC no. (if applicable)_________________________
Complaint reference and date:_______________________________
Complaint Details:______________________________
Preliminary Investigation details_____________________
Regards,
(Quality Head)
Attachment – 7: Format of Extension Note for investigation of Complaint.
Extension Note No. ______________ Date : ______________
Ref. Location Complaint No. ___________________ Ref. CQ Complaint No. : _____________
Ref. third party Complaint No. : _____________
Initial Target Completion date : __________________
Current status of Investigation :___________________________________________
Pending activities for Investigation (as per action plan) :______________________
Reason for delay :__________________________________________________
Justification for Delay :_______________________________________________
Revised target Date :__________________
Prepared by: Reviewed by Department Head Reviewed by QA
Approved by Quality Head
Attachment – 8: Format of Market complaint Investigation Report.
Location complaint No._________________________________
CQ complaint No. _________________________________
Reference Complaint no. and date _________________________________
Product _________________________________
Batch No._________________________________
Mfg. date _________________________________
Exp. Date_________________________________
Pack style and NDC No. If any _________________________________
Market Complaint details_________________________________
Complainant_________________________________
History _________________________________
Other details _________________________________
Qty. of complaint sample _________________________________
Physical inspection of complaint sample_________________________________
Physical inspection of the control sample_________________________________
Tests – performed on complaint sample _________________________________
Analytical results of complaint sample _________________________________
Tests – performed on the control sample _________________________________
Analytical results of the control sample _________________________________
Evaluation of processing parameter trend _________________________________
In-process analytical trend _________________________________
Evaluation of FP analytical trend _________________________________
Review of stability data_________________________________
Batch document _________________________________
Review of other related documents (like a sequential log of m/c etc) _____________________
Review of trial batch results _________________________________
Attachments _________________________________
Interpretation / Probable cause _________________________________
Impact on current batch / Other batches of the same product / Other products__________
Immediate Action taken_________________________________
Corrective action is taken / to be taken with the target completion date ________________
Preventive action is taken / to be taken with the target completion date ______________________
Conclusion _________________________________
Prepared by _________
Reviewed by Production / Packing Head__________ Reviewed by QA_______
Approved by QA Head____________
Approved by (Quality Head) ____________ Approved by (Location Head)____
Level – I Closure on ___________ by ___________.
Level – II Closure on ___________ by ___________.
Attachment – 9: Format of Register for Extension Note.
| Sr. No. | Date | Reference complaint number. | The initial Target date for completion | Proposed Date for completion | Extension Note Number | Approved on | Sign / Date |
Remarks |
Attachment – 10: Flow chart showing types & definitions of the market complaint.
Attachment – 11: Flow chart for the handling of the market complaint.
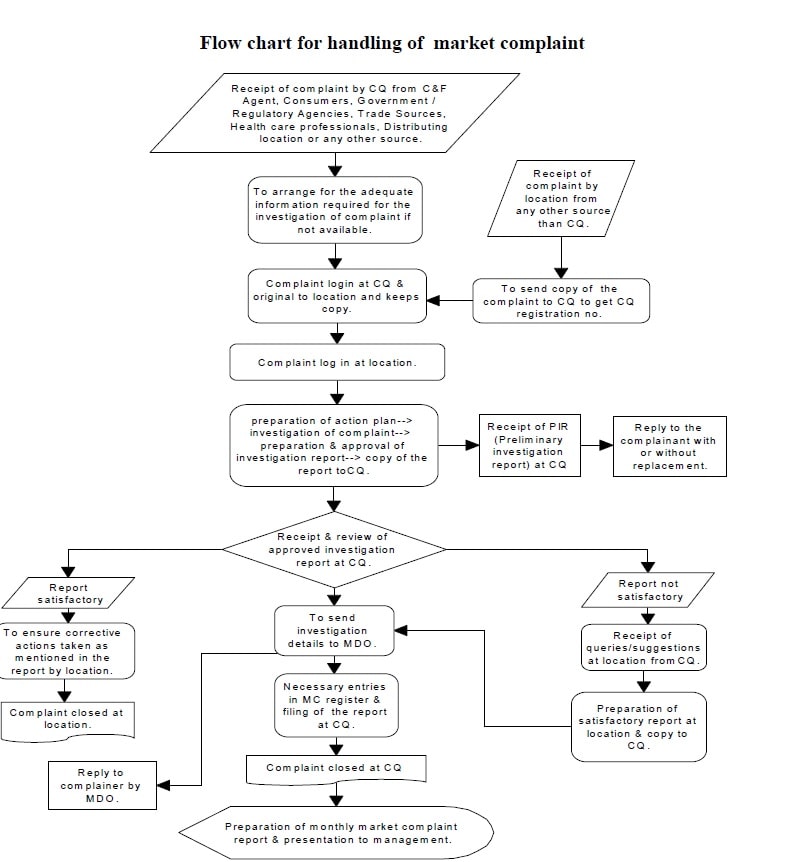
-
- Annexure
-
- Annexure – 1: Flow chart for the handling of complaints received at the Distribution location.



Pingback: Pharmacovigilance - Market Complaint (EU) - Pharma Beginners
Pingback: Dietary Supplements Recall Guideline - Pharma Beginners
Pingback: Out of Specification Result in Microbiology - Guideline - Pharma Beginners