Standard Operating Procedure (SOP) cum Guideline for Good Documentation Practices for cGxP documents (Electronic and Handwritten) Pharmaceutical Drug Manufacturing Plants.
Guideline for Good Documentation Practices
1.0 PURPOSE:
-
- This Standard Operating Procedure (SOP) defines the requirements for the compliant, consistent, and accurate completion of Good Documentation Practices.
2.0 SCOPE:
-
- This standard applies to cGxP documents (electronic and handwritten) used in the manufacturing, packaging, labeling, testing, storage, and distribution of Drug Products.
3.0 REFERENCES:
-
- SOP for Recording of Specimen Signatures of Employees
-
- In house
4.0 RESPONSIBILITY:
-
- Quality head/designee shall be responsible for implementing and maintaining procedures to provide requirements for good documentation practices.
-
- QA shall be responsible for implementing and managing a master signature log of all department personnel, including contract and temporary personnel.
-
- QA head/ designee shall be responsible for Managing investigations and impact assessments for deviations/incidents concerning Good Documentation Practices / GDP procedures.
5.0 ABBREVIATIONS:
-
- CC No: Change Control Number (SOP for Change Control Management)
-
- PDF: Portable Document File
-
- GDP: Good Documentation Practices
-
- SOP: Standard Operating Procedure
-
- FAX: Far Away Xerox
-
- RA: Regulatory affair
-
- COA: Certificate of analysis
-
- NA: Not Applicable
6.0 DEFINITION:
-
-
Authorized By:
-
-
- The signature of the person responsible for providing official permission or approval to another individual to perform a particular task.
-
-
Data Governance:
-
-
- The total of arrangements to ensure that data, irrespective of the format in which it is generated, is recorded, processed, retained, and used to ensure a complete, consistent and accurate record throughout the data lifecycle.
-
-
Data Integrity: (Related: SOP for Handling of Data Integrity Observations)
-
-
- The extent to which all data are complete, consistent, and accurate throughout the data lifecycle.
-
- Data Lifecycle:
-
- All phases in the life of the data (including raw data) from initial generation and recording through processing (including transformation and migration), use, data retention, archive/retrieval, and destruction.
-
-
Document:
-
-
- A record that describes how an activity, event, or process was actually performed.
-
- Documentation:
-
- A reliable (written) record that can be used at a future time to clearly and completely recreate an activity, event, or process. (Good Documentation Practices)
-
-
Electronic Record:
-
-
- A combination of text, graphics, data, audio, pictorial or other information represented in digital form that is created, modified, maintained, archived, retrieved, or distributed by a computer system.
-
-
Good Documentation Practices:
-
-
- A systematic procedure of preparing, reviewing, approving, issuing, recording, storing, and archival of documents associated with GxP activities.
-
-
Instruction Document:
-
-
- A document, which gives general or specific instruction on how to perform certain activities (i.e. SOP, Standard Test Procedure).
-
-
Master Document:
-
-
- An approved set of instructions, which can be replicated to issue controlled operational copies.
-
-
Metadata:
-
-
- Metadata is data that describes the attributes of other data and provides context and meaning.
-
- Typically, these are data that describe the structure, data elements, interrelationships, and other characteristics of data.
-
- It also permits data to be attributable to an individual.
-
- Examples of metadata might include a user name, date, and time.
-
-
Original Record:
-
-
- Data as the file or format in which it was originally generated, preserving the integrity (accuracy, completeness, content, and meaning) of the record, e.g. original paper record of manual observation or electronic raw data file from the computerized system.
-
-
Performed by:
-
-
- Signature of the person performing the work being recorded.
-
-
Raw Data:
-
-
- Original records and documentation retained in the format in which they were originally generated (i.e. paper or electronic) or as a “true copy.”
-
- Raw data must be contemporaneously and accurately recorded by permanent means.
-
- In the case of basic electronic equipment that does not store electronic data or provides only a printed data output (e.g., balance or pH meter), the printout constitutes the raw data.
-
-
Record:
-
-
- Any document, which contains recorded evidence of an activity completed during manufacturing, testing, and release of a product.
-
- Records can be handwritten or electronically generated.
-
- Examples of Records include maintenance histories, validation reports, change control records, laboratory notebooks, stability reports, and batch records.
-
- Reviewed by:
-
- Signature of the person certifying the accuracy and completeness of the document.
-
-
Significant/Critical Steps in GMP Process:
-
-
- Those steps in a manufacturing or packaging process that are required to be checked by a second person either as defined by regulatory requirement or as a good manufacturing practice required by Batch Record, Protocol, or other GMP documentation to verify that they have been properly executed as prescribed by procedure.
-
- True Copy:
-
- An exact verified copy of the original record.
-
-
Verified by/Checked by:
-
-
- The signature of the person responsible for witnessing or conducting an independent check to ensure the operation, test, inspection, calculation, or other actions followed required instructions and procedures and for verifying entries in the record made by the person performing the task.
7.0 PROCEDURE – GOOD DOCUMENTATION PRACTICES:
-
General Requirements for Good Documentation Practices
- All GxP documents shall be accurate, contemporaneous, legible, and permanent, truthful and complete, readily retrievable, and traceable.
-
- Data integrity shall be given utmost importance in Good Documentation Practices.
-
- All GxP activities shall be carried out with valid, correct and current effective versions of instruction documents and recording formats.
-
- Controlled documents shall have a unique identification number and a version number. The instruction source and unique identifier shall be documented in the respective record.
-
- All documents/records shall have page numbers (preferred format: “Page X of Y”).
-
- All Master Documents shall be typed or preprinted. Handwritten documents shall not be used as Master Documents.
-
- Instruction Documents shall have the effective date printed, handwritten or stamped on them.
-
-
-
Date Format :
-
-
-
- The date shall be written in the following formats:
DD/MM/YY For example 25/08/20
Where: DD is denoted to date, MM is denoted to month, YY stands for last two digits of the year
-
- All documentation of time and verification of time and date stamps shall be performed using a consistent source, i.e. a Slave Clock system where all clocks in production, lab and packaging areas depend on a master clock to assure uniformity of performance.
-
- Time generated from all equipment and computers used for GxP activities shall be synchronized with the company clock provided in the area.
-
- Verify the time from the company clock provided in the area where the activity is being performed and not from personal watches.
-
-
Time Format
- Record the time in the following formats:
-
-
-
- For all the GMP records change the date after 24 hours cycle daily, i.e. after 23:59:60 or 00:00:00 in the night as per Indian Standard Time.
-
-
-
- Time: Write time numerically in the form of HH:MM or HH:MM:SS, as applicable, in the document using 24 hours cycle daily. e.g.,
-
For 03:45 AM write 03:45 or 03:45:00
03:45 PM write 15:45 or 15:45:00
12:30 AM write 00:30 or 00:30:00
-
- Time duration to be written as shown below:
-
-
- Note 1) If for some process the observed time duration is 2 minutes and 30 seconds then it should be written as 2min 30 sec and not as 2:30min.
-
-
-
- 2) In case time is printed from a machine or a computer, the time format of the machine or the computer shall be followed.
-
-
- Attachments shall be cross-referenced to the parent document and the parent document shall be cross-referenced to the attachments.
-
- In case of electronic records, all child records of a parent document shall have an indication of the relationship with the parent document.
-
- Data shall be recorded directly on approved and authorized formats only (e.g., batch production records, batch packaging records, laboratory notebooks, raw data sheets).
-
- Data for GxP documentation shall not be recorded on unauthorized documents.
-
- All GxP documents shall identify the significant steps that require checks by a second person while performing the activity (e.g. witnessing dispensing materials for batch production).
-
- Impermanent records like data printed on thermal paper, thin layer Chromatography (TLC) etc., shall be copied onto a permanent medium and the copies shall be attached to or stored along with, the original signed records.
-
Handwritten Documents- Good Documentation Practices:
-
- Use only indelible ink (preferably only black or blue colors) in GxP documents.
-
- The ink used shall be such that it can be photocopied.
-
- Write all GMP records only with a permanent BLACK indelible ink pen.
-
- Use permanent BLUE indelible ink pen for the signing of master documents for all departments.
-
- QA/QC supervisor shall used BLUE indelible ink pen for checking and approval only.
-
- The QA supervisor shall use the permanent BLUE indelible ink pen to sign all GMP records, checking or approving the data.
-
- All entries should concise, legible, unambiguous and accurate.
-
- Sign on Handwritten records or initialed and dated at the time the information is entered.
-
- All entries related to experiments being performed shall be done in chronological order.
-
- In the case of continuous pages of a notebook that are not being used to record data, continuity shall be denoted by recording the reference of the notebook number or page numbers at appropriate places.
-
- For example, if an experiment is recorded in a laboratory notebook on Page 25 and calculations are recorded on Page 35, a cross-reference linking the experiment with the calculations shall be recorded on both pages.
-
-
The following practices are strictly prohibited under Good Documentation Practices:
-
-
- Use of ditto marks (“) or down arrows ( ) to fill in repetitive entries.
-
- Use of pencil or any removable/water-soluble ink.
-
- Eraser or ink remover use.
-
- Use of “white-out or correction fluid” to cover recording then write over it.
-
- Use of a stamp to replace manual dating, initials or signature on GMP documents, except in the case of validated electronic signature.
-
- When one option is to be selected from several text options, the correct option shall be preferably marked with “√”. (Good Documentation Practices)
-
- An example is shown below:
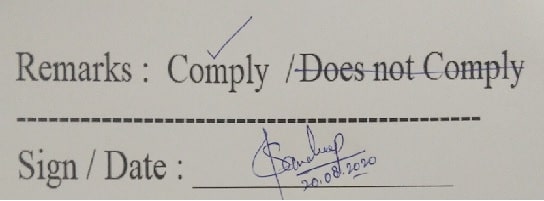
-
- Working with Blank or Unused page/space:
-
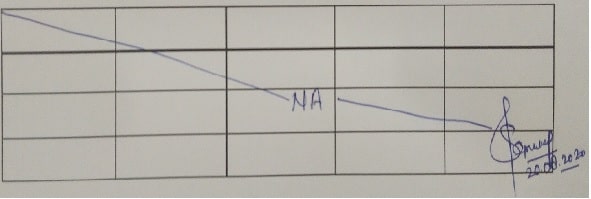
- Do not leave blank space in any GMP record.
-
- Blank spaces or pages shall have a single line through them with a signature and date and the reason for the page is blank (e.g. “Not Applicable”, “NA” or “N/A”).
-
- An example is shown below:
-
-
Signature Practices under Good Documentation Practices:
- QA shall maintain an updated master signature log wherein each employee involved in GxP activities shall provide their signatures and initials.
-
-
- Use the log for traceability of signatures for GxP records as per SOP Recording of Specimen Signatures of Employees.
-
- Signatures for all GxP activities shall always be accompanied by the relevant date wherever a separate date column has not been provided.
-
- Signatures indicate that the Signatory is responsible for the accuracy of data and information for the activity being signed for.
-
- The Signatory shall confirm the accuracy and completeness of information and data before signing.
-
- Persons preparing, reviewing, or approving documents or persons recording, verifying, or approving records shall sign and write the current date in the documents.
Note: Current date refers to the date when the document/record is signed.
-
- All document signatories shall be adequately trained for the activity performed by them.
-
- For each activity/document (as applicable), each person shall sign (with current date) either as a Doer or a Verifier (also called “Checker”) or a Reviewer or an Approver.
-
- One person shall not sign for multiple roles for the same activity or entry (e.g. a doer cannot be the “Verifier”/ “Reviewer”/”Approver” for the same activity or entry recorded).
-
- No employee is authorized to sign for an activity performed by another employee.
-
- A clear meaning of each signature shall be provided (e.g. “Performed By”/”Verified By”/”Reviewed By”/”Approved By”).
-
-
Definitions/Significance of Signatures:
-
-
- Prepared By / Performed By / Written By / Analyzed By (also called “Doer”): The signature of the person actually carrying out the operation, test, inspection, calculation, or other actions.
-
- Entries in the documents/records along with Signature and Date shall be made at the time when the activity is performed (contemporaneously).
-
- The signature of the “Doer” denotes that the “Doer” has performed the activity and confirms the authenticity of the data as that of the activity performed.
-
- The Doer shall also check the result for its compliance against the specified limits/acceptance criteria and is expected to inform the respective Supervisor/Team Lead/Manager in case the results do not comply.
-
- Verified By/Checked By: The signature of the person responsible for witnessing or conducting an independent check to ensure the operation, test, inspection, calculation or other actions followed required instructions and procedures and verifies the entries made by the Doer.
-
- The “Verifier”/”Checker” shall record and sign concurrently for the ongoing activity being checked.
-
- The signature of the “Verifier” denotes that the Verifier has confirmed that the entries are made correctly and are complying with predefined specifications/acceptance criteria.
-
-
Reviewed By:
-
-
- The signature of the person responsible for examining the documentation and certifying that the document/record was prepared/filled appropriately and in compliance with requirements.
-
- The “Reviewer” shall review and sign (with date) for the activity/document/record being reviewed; the reviewer may or may not be present when the activity is being performed.
-
- The “Reviewer” shall review the completeness of the document/record and conformance of results recorded during the activity to established process parameters, limits, and other applicable standards that define requirements of the activity being performed.
-
- The signature of the “Reviewer” denotes that the document/record has been examined, all requirements have been fulfilled and the document/record demonstrates that the process was followed in accordance with the instructions provided.
-
-
Approved By:
-
-
- The signature of the person accepting the document/record for conformity to requirements.
-
- The “Approver” shall review and sign (with date) for the activity/documents/record being approved; the Approver may or may not be present when the activity is being performed.
-
- Review the conformance of results recorded during the activity to established process parameters, limits, and other applicable standards that define requirements of the activity being performed.
-
- The Signature of the “Approver” denotes that the document/record demonstrates that the process was followed in accordance with the instructions provided and is approved for conformity with requirements.
-
-
Authorized By:
-
-
- The signature of the person responsible for providing official permission or approval to another individual to perform a particular task.
-
-
Designee:
-
-
- Reviewers/Approvers may delegate authority to another suitably qualified person to review/approve records, as applicable.
-
- The designee shall be qualified to perform the delegated task based upon relative job position, training, experience, and subject matter expertise.
-
- Though a designee may perform the delegated task (of reviewing/approving, as applicable), final accountability of the activity performed by the designee shall reside with the person delegating the task.
-
- Supervisors of a signatory and/or members of the same department at an equivalent or higher titles may function as designees without prior delegation of authority.
-
-
The following signature practices are strictly prohibited:
-
-
- Pre-dating or post-dating (backdating) either documents or corrections.
-
- Pre-dating is completing an activity and then signing/dating that the activity was performed at a later time/date.
-
- Back-Dating is completing an activity and then signing/dating that the activity was performed at an earlier time/date.
-
- Signing someone else’s name unless the signer is a Designee and it is clearly notated that the Designee has signed on behalf of the person, e.g., if Ramesh Kumar is a designated signer for Suresh Kumar, then Ramesh Kumar would sign as, “Ramesh Kumar for Suresh Kumar,
-
-
Correction of Errors/Handling of Missed entries:
-
-
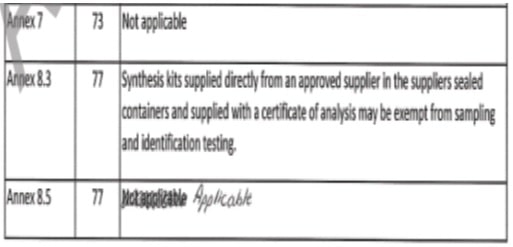
- DON’Ts: Entries in documents/records shall not be canceled, erased, obliterated, or otherwise rendered illegible by using correction fluid/tape, overwriting, or crossing out with multiple strokes.
-
- Example: Overwriting / Obliterating/Crossing Out
-
- This is an unacceptable correction

-
-
DOs: (Good Documentation Practices)
-
-
- When a correction is necessary, the erroneous/wrong entry shall be crossed out with a single horizontal line such that it shall not obscure the original entry.
-
- A brief reason for the correction shall be noted as to why the change was made and the correction shall be signed and dated.

-
- Example:
-
- This is an acceptable correction as the original information must still be legible after the correction is made.
-
- When the reason for change requires a lengthy explanation, it shall be clearly stated and shall be justified by supporting rationale.
-
- The reason may be in the form of a memorandum that is referenced in and attached to the original record.
-
- If this change affects the outcome of data, an investigation shall be initiated and, post-investigation, correction of the error shall be made and the change shall be countersigned by a supervisor.
-
-
Supporting documents shall be attached if required.
-
-
- If there is insufficient space to enter a remark, then an annotation mark shall be placed near the incorrect entry and explained on the same page along with signature and date.
-
- Attempts to cover up mistakes are serious data integrity concerns and are strictly prohibited at all levels.
-
- The following conditions that may occur during correction of errors/completion of missed entries shall require evaluation as per the current SOP of Investigation.
-
- The employee who made the error/person who missed recording data is not available in the organization.
-
- The information for an error/missed entry cannot be traced or determined.
-
- Errors/Missed Entries identified after a document/record has been approved/closed by QA.
-
- The requirement for correction of errors, including transcription/typographical errors related to data /missed entries in documents/records, has already been submitted to Regulatory Agencies.
-
- Errors identified in approved documents while the activity is being performed.
-
-
The following general rules shall be followed for correction of errors/handling of missed entries:
-
-
- All error corrections/filling of missed entries shall be done by the document “Doer”, irrespective of the time/date at which the error was noticed.
-
- If a worker (the “Doer”) made an error/missed an entry and they are no longer available due to reasons, such as leaving the organization or taking a leave for an extended period, such matters shall be escalated to the Department Head and an investigation shall be initiated.
-
- Based on the impact assessment and investigation outcome, another employee may be authorized to correct the error/fill in the missed entry as part of the corrective action.
-
- The employee shall provide adequate justification and mention the name of the doer while performing the correction.
-
- Not all missed entries can be filled (corrected); if the information for filling in the missing data cannot be traced or determined, the Functional Manager or designee and QA Manager shall be informed immediately and shall take steps for further actions (including a decision for not filling in the missing entry) and provide explanations, which shall be recorded.
-
- The corrections/ explanations shall be countersigned by the QA Manager or designee.
-
- An investigation shall be completed and used by QA to determine the disposition of the impacted products.
-
-
Other Good Practices under the Documentation
- Errors/Missed Entries identified at the time of verification/ review/approval of a document/record may be managed at the level of verifier/reviewer/approver, as applicable; that is, the doer may correct the erroneous entry/fill in the missed entry and mark it as “Error Corrected”/”Late Entry” (as applicable) and sign (with current date) in the presence of the Verifier/Reviewer/Approver, as applicable.
-
-
- Errors/Missed Entries identified after a document has been approved/closed by QA shall be corrected/filled in (as applicable) by the doer only in the presence of QA and QA shall counter-sign near the correction.
-
- Typographical Errors/Missed Entries observed in “approved” documents during activity, shall be corrected/filled in (as applicable) on the respective page by the concerned supervisor, including signature and date and shall be verified by the QA Manager/designee.
-
- The error shall be corrected by putting the correct stamp imprint adjacent to the incorrect one.
-
- The incorrect stamp imprint shall be struck off by “Doer” with a single horizontal line in a manner that it shall be readable and not obscured.
-
- The “Doer” shall sign with a date near the crossed-out incorrect stamp imprint providing a rationale /justification; this activity shall be verified and signed (with date) by QA.
-
-
Analytical/Manufacturing Documentation:
-
-
- Production officer and QC Analysts shall record actual results obtained at the time of performing an activity, without bias or prejudice.
-
- Only validated Excel spreadsheets shall be used for calculations. Wherever such Excel spreadsheets are not available, calculations shall be re-verified with qualified calculators.
-
- During the manufacturing process, sequential steps listed in the MI shall be directly recorded in the batch records as soon as the activity is performed.
-
-
- In the case of laboratory analysis, step-by-step details of the
-
-
-
- Testing procedure,
-
-
-
- Dilutions,
-
-
-
- Critical test parameters, and date/time of activity,
-
-
- As required by the Standard Test Procedure (STP), shall be documented concurrently in Analytical template/worksheet/Laboratory notebooks or using controlled forms.
-
- A description of the sample received for testing with identification of the source, quantity, lot number, or other distinctive code, date sample was taken and date sample was received for testing shall be documented in the sample notebook or equivalent.
-
- All weight slips, printouts, chromatograms, spectrum, records of analysis, etc., shall be signed with the date by the person performing the activity immediately after completing the activity and included with the respective record.
-
-
Recording of Raw Data (Good Documentation Practices):
-
-
- If raw data prints/slips are too small to be included with the records
-
- They shall be affixed on the specified stationary/space designated for them.
-
- These affixed printouts shall be cross-referenced to the parent documents and shall be enclosed with the parent record.
- All invalidated/disregarded chromatograms and other cGxP documents (Good Documentation Practices) shall have supporting justification written by the Analyst performing the activity, be signed/dated, and approved by relevant stakeholders.
-
- Wherever data/information for an activity must be captured electronically by an automated system (e.g. PLC, Datalogger), the parent document shall contain instructions to attach copies of such printouts.
-
- All elements needed to associate the electronic records with the analysis and/or study shall be fully documented.
-
- These printouts shall be signed and dated.
-
- In such cases, the signature represents that the person performing the activity has verified that the printout is accurate and a complete reproduction of data/information taken from the electronic system.
-
- Readings or values that are to be recorded from digital electronic displays shall be transcribed as they appear from the system to documents.
-
-
Example:
-
-
- If a balance is displaying 23.230 kg, then the value shall be recorded as 23.230 kg and not as 23.23 kg.
-
- In case a sample has been analyzed by two or more Analysts for different tests, each Analyst shall complete the test and related documentation for respective tests and sign (with date) his or her part.
-
- After ensuring the completion of all tests required per specification, including those sent to the contract laboratory for certain tests, the COA shall be prepared.
-
- Entries like “Complies/Does not comply” only allowed for the binary observations but the binary observation shall be specific. e.g. Limit test shall mention the observation noticed and TLC shall mention the comparison with the spot.
-
- Concordance of another analyst shall be taken for observations of subjective tests. e.g. limit tests, TLC plates, etc. Both the analyst shall initialize and sign the observation.
-
- Printouts from the instruments relevant to the analysis shall be retained and no such document shall be discarded even if they are not of use in the calculation.
-
-
Rounding – off of values: (Related: SOP for Rounding off Test Results)
-
-
- The rounding off values is applicable only to the calculations and not to the observed readings.
-
- Limits which are fixed numbers, shall not be rounded off.
-
- e.g., If the limit is 2 – 8 °C, then observed value 8.2 cannot be rounded off.
-
- When rounding off of any value is required, follow the below procedure.
-
- If the last digit equals to or greater than 5, it is eliminated and the preceding digit is increased by one.
-
- If the last digit is smaller than 5, it is eliminated and the preceding digit remains unchanged.
For examples see the below Table – 1:
| Illustration of Rounding of Numerical Values for comparison with Requirements | ||
| Requirements | Unrounded Value | Rounded Value |
| Product Yield Limit
(99.0% to 101.0%) |
98.926 % | 98.93 % |
| 100.124 % | 100.12 % | |
| 99.655 % | 99.66 % | |
-
- Assigning Due Date:
-
- For assigning Due Date in all GMP records, calculate due date as per frequency for that particular activity from the day on which that activity is performed.
-
- The status of the activity can be valid up to the due date.
-
- For example, consider the case of assigning a due date for re-cleaning of prefilter for which cleaning frequency is 15 days. (SOP for Good Documentation Practices)
-
- Suppose the cleaning of pre-filter is done on 01/03/20 since the frequency of prefilter is 15 days,
-
- The due date for re-cleaning of prefilter can be assigned 15/03/20 and cleaning can be considered valid up to 15/03/20.
-
- Record all information in legible handwriting in all GMP records.
-
- Some typefaces have characters that are easily misread and create confusion.
-
-
General Requirements of Good Documentation Practices:
-
-
- GxP documents (Good Documentation Practices) shall provide a clear, accurate history of an activity or event.
-
- Procedures shall require that all entries in GxP documentation (Good Documentation Practices) be permanent, legible, accurate, prompt, clear, consistent, complete, direct and truthful.
-
- Only current approved versions of SOPs or forms shall be used to perform cGxP activities.
-
- Systems shall be in place to prevent the use of superseded documents.
-
- Uncontrolled documents (Good Documentation Practices) shall not be used in GMP areas.
-
- Facsimile (Fax) can be used for GMP activities but must be signed/dated to do so.
-
- In addition, the Fax shall be signed/dated by the recipient upon receipt.
-
- Email from non-validated or unsecured systems should not be used as the primary document where a hardcopy is required.
-
- When there is no alternative, the responsible person should print out the email, sign, and date as being received.
-
- Email may be used to confirm receipt of GMP documents in accordance with the requirements of this section.
-
- Documents in PDF (or other image formats) sent as an attachment to an email can be treated as GMP similar to a Fax.
-
Original records shall be kept in a secure location with controlled access.
- Original records (Good Documentation Practices) shall be stored with the batch documentation and archived by the respective documentation cell.
-
- Copies of master documents / raw data shall not be allowed unless justified.
-
- When copies of master documents / raw data are required, for example during a regulatory inspection, they should be clearly identified as an “UNCONTROLLED COPY”.
-
- Use of scrap paper, post-it notes or loose (unbound) paper in GMP areas to record data is not permitted.
-
- When printouts are made that require verification, e.g. proof of weight printout) and are not automatically verified by a validated electronic system, they shall sign/dated manually by personnel performing the work (Doer) and the person verifying the work, as required.
-
- Procedures shall require that batch records include identification of the persons performing and directly supervising or checking each significant step in the operation.
-
- Reviews to ensure documentation is complete and accurate shall be performed by a qualified individual who did not perform the task.(Good Documentation Practices)
-
- Procedures shall require that reference to other GMP documents/records (i.e. deviation, discrepancy, CAPA or investigation numbers) shall be documented in the appropriate step of the document or in the comment section of the document.
-
- The following elements shall be included, as applicable, when documenting a comment or event on a GMP document/record:
-
-
- What happened
-
-
-
- When it happened (time)
-
-
-
- Where it happened
-
-
-
- Personnel involved
-
-
-
Good Documentation Practices Requirements (Applicable For Laboratory Records):
- All data generated within the laboratory shall be recorded.
-
-
- The intent/objective of the testing shall be recorded prior to the initiation of data acquisition.
-
- The requirements of the testing shall be covered by a specification, validated/qualified method, protocol or investigation.
-
- Laboratory records shall meet the regulatory requirements.
-
- The requirements herein are applicable to both hardcopy and electronic records.
-
-
Clearly Written Documentation –
-
-
- All documents shall be accurate and recorded in a manner that prevents errors and ensure consistency. Sufficient space shall be provided for entries.
-
- If multiple documents or records are used in parallel for documentation,
-
- Then each shall reference the other and be traceable by formal documentation numbers or record identification.
-
-
Pagination –
-
-
- Unbound documents shall have page numbers, such as page XX of YY, to indicate the total number of pages in the document.
-
-
Content:
-
-
- Laboratory records shall include complete data derived for all tests necessary to assure compliance with established specifications and requirements, including examinations and assays.
-
-
Sample:
-
-
- A description of the sample received for testing with identification of the source, quantity, lot number, or other distinctive code, date sample was taken and date sample was received for testing.
-
- Date/time: Where required, the date/time of activity shall be documented accurately.
-
-
Electronic Records (Good Documentation Practices):
-
-
-
Electronic records shall include elements for control of the process including but not limited to:
-
-
-
- Implementation of an overarching data governance system,
-
-
-
- Ensuring data integrity arrangements,
-
-
-
- Appropriate management of the data lifecycle, and use of an audit trail.
-
-
-
Systems shall be designed to assure compliance and data integrity including but not limited to:
-
-
-
- Restricting control/access to templates used for recording data.
-
-
-
- Implementing user access rights that prevent amendments to data or audit trail information.
-
-
-
- Ensuring access to raw data for staff performing data checking activities.
-
-
-
- Using automated data capture or printers linked directly to the equipment.
-
-
-
- Locating printers in close proximity to associated activities.
-
-
-
- Using clocks for recording timed events.
-
-
-
- Having persons performing observed task countersign record whenever possible.
-
-
-
- Recording the execution of critical operations contemporaneously by the user in single electronic transactions not combined with other operations.
-
-
-
Audit Trail (Good Documentation Practices):
-
-
- When electronic records are used to capture, process, report or store raw data the system design should ensure retention of full audit trails, showing all changes to the data while retaining previous and original data.
-
- All changes made to data should be associated with the person making those changes, including a timestamp and reason for making the change.
-
- Users shall not have the capability to disable the audit trail function.
-
- Audit trail review shall be included as part of the routine GMP data review/approval process and should be documented.
-
- QA should periodically review a sampling of relevant audit trails, including raw data and metadata, as part of the self-inspection procedures to ensure data governance compliance.
-
-
Test Method (Good Documentation Practices):
-
-
- A statement of each method used in the testing of the sample.
-
- The statement shall indicate the location of data that establishes that the methods used in the testing of the sample meet proper standards of accuracy and reliability, as applied to the product tested.
-
- The method source and unique identifiers (e.g. version, issue date, etc.) shall be documented.
-
- Complete records shall be maintained of any modification of an established method employed in testing.
-
- Such records shall include the reason for the modification and data to support the valid use and shall align with site-specific change control procedures.
-
-
Specification and/or acceptance criteria:
-
-
- The identification of a specification and/or acceptance criteria associated with the analysis or study shall be fully identified
-
- For Example :
-
-
- Specification number,
-
-
-
- Version number,
-
-
-
- Specification name, and effective date or
-
-
-
- Protocol number,
-
-
-
- Version number,
-
-
-
- Protocol name, and
-
-
-
- Approval date).
-
-
-
Standards, Reagents, and Solutions (Good Documentation Practices):
-
-
- Complete records shall be maintained of all testing and standardization of laboratory reference standards, reagents, volumetric solutions and standard solutions.
-
- Name, source, grade, date of receipt, expiration date and lot or unique identifier shall be provided for each.
-
- Data on or cross-reference to, the preparation and testing of reference standards, reagents and standard solutions.
-
- For quantitative standards, the actual strength (property value or calculation value) shall be recorded.
-
-
Weights:
-
-
- A statement of the weight or measure of samples, standards, and reagents used for each test, where appropriate.
-
- Weights taken shall be supported by balance printouts detailing the balance used, weights measured, time, and date.
-
- Weighs for individual dosage units tested for Content Uniformity and Dissolution Testing can be captured, even though they are not required for calculations.
-
- These weights can be very useful for supporting data, especially during laboratory investigations.
-
-
Printouts, Graphs, Charts, and Spectra:
-
-
- A complete record of all data secured in the course of each test, including
-
-
- All printouts,
-
-
-
- Graphs,
-
-
-
- Charts and
-
-
- Spectra from laboratory instrumentation, properly identified to show the specific component, drug product container, closure, in-process material, or drug product and lot tested.
-
- Any printouts, graphs, charts, or spectra shall be appropriately identified, signed, and dated and properly retained and crossed referenced.
-
- Where practical, they shall be permanently affixed (with glue or tape) to the notebook or controlled datasheet and be marked such that it would be obvious if they become separated (e.g. marked on a border).
-
- Otherwise, all individual pages of a data set shall be maintained and secured together as a packet preventing the intentional or unintentional misplacement of the individual pages.
-
-
Laboratory Instruments, Apparatus, Gauges, and Recording Devices:
-
-
- Complete records shall be maintained of the periodic calibration of laboratory instruments, apparatus, gauges, and recording devices.
-
- Records shall include validations, calibrations, maintenance, and cleaning or repair operations.
-
- Instrument logs can be used to record the daily instrument performance verification check in addition to any instrument incident and unscheduled repairs.
-
- All pertinent instrument parameters need to be documented (Good Documentation Practices).
-
-
Calculations:
-
-
- A record of a calculation example and all calculation factors in connection with the test, including units of measure, conversion factors, and equivalency factors shall be documented.
-
-
Results:
-
-
- There shall be a statement of the results of tests and how the results compare with the established specifications or acceptance criteria of identity, strength, quality, and purity for the component, drug product container, closure, in-process material, or drug product tested.
-
-
Signatures and date:
-
-
- The initials or signature of the person who performs each test and the date(s) the tests were performed.
-
- Each notebook/worksheet/template/form page shall be dated with a start date and signed and dated on completion of the page; or if not completed, at the end of the scheduled workday.
-
- The initials or signature of a second person and the review date showing that the original records have been reviewed for accuracy, completeness, and compliance with established standards.
-
-
Electronic Records and Electronic Signatures:
-
-
- Persons who use closed systems to
-
-
- Create,
-
-
-
- Modify,
-
-
-
- Maintain or
-
-
- Transmit electronic records shall employ procedures and controls designed to ensure the
-
-
- Authenticity,
-
-
-
- Integrity, and,
-
-
-
- When appropriate,
-
-
- the confidentiality of electronic records and to ensure that the signer cannot readily repudiate the signed record as not genuine.
-
- Signature manifestation information should be subject to all controls required for electronic records and should include the following:
-
-
- The printed name of the signer.
-
-
-
- Date and time signature was executed.
-
-
-
- The meaning associated with signature (e.g. review, approval, authorship).
-
-
-
Retention of Documents:
-
-
- All GxP documents shall be retained for a pre-designated period.
-
- Following the expiration of the retention period, GxP documents shall be destroyed in a controlled manner
Related: SOP for Document Management System



Pingback: AlCOA and ALCOA Plus Principles for Data Integrity -
Pingback: How to make an effective training documentation? - What Type Degree
Pingback: Out of Specification Result in Microbiology - Guideline - Pharma Beginners
Pingback: Environmental Monitoring Guide - Non Sterile Facility - Pharma Beginners