Standard Operating Procedure (SOP) for Training Management of newly joined as well as an existing employee (staff and workers) working in a cGMP environment in pharmaceuticals.
Procedure for Training Management
1.0 PURPOSE:
-
- The purpose of this SOP is to describe the procedure to provide the guideline for training (TRN) of employees.
2.0 SCOPE:
-
- This procedure is applicable to the pharmaceutical company for the different types of training (TRN) as listed below:
Note: Word “TRN” being used at the place of Training (Abbreviation: TRN=Training)
-
- Induction TRN

- Induction TRN
-
- Job-specific department TRN
-
- Unplanned TRN
-
- On the Job TRN (OJT)
-
- Classroom TRN
-
- External TRN
-
- Retraining
-
- This SOP covers TRN activities for personnel, whose roles and responsibilities could affect the SISPQ of the Product.
3.0 REFERENCES:
-
- SOP for Assigning of Job Responsibility
4.0 RESPONSIBILITY:
-
-
Individual Employee shall be responsible for –
-
-
- Maintain, update their individual TRN file and Employee TRN summary sheet (as and when required).
-
- Individual Employee shall submit their TRN file to P&A Dept. at the time of resigning/ transfer.
-
-
Site Training coordinator shall be responsible for-
-
-
- Issuance TRN file to the new employee, identify individual TRN need by co-ordination with the section heads and ensure induction TRN given to the newly joined employee.
-
- Providing ethical quality pledge format and site QA Head or designee conducts the ethical quality pledge to newly join employee,
-
- Review induction TRN report of newly joins employee after completion of induction TRN.
-
- Preparation and updating of the list of certified trainers and issuance of the SOP matrix.
-
- STC shall be involved in the certification procedure of trainer, prepare central TRN calendar, coordinate and impart TRN as per the TRN calendar
-
- Individual Dept. training coordinator shall be responsible for issuance of employee TRN summary sheet and maintain TRN summary sheet issuance register.
-
-
All Dept. Head / Designee shall be responsible for-
-
-
- Identify TRN topics, to review the induction TRN, to prepare annual training calendar and compliance of SOP.
-
- Certify new employees for doing their job as per their job responsibilities and ensure TRN to be imparted as per SOP.
-
- Head shall ensure that no work shall be assigned to Employees or Contract Employees until the required TRN is imparted.
-
-
Respective Dept. Trainer / Training Coordinator shall be responsible for-
-
-
- Assure and impart TRN as per SOP, training to be given as per SOP, maintain TRN Number Allocation Log, and issue TRN number.
-
- Prepare the questionnaire for evaluation, to evaluate the questionnaire after TRN session completion and to evaluate the effectiveness of TRN.
-
- Head QA/ Designee shall ensure TRN to be given as per SOP, approve Induction TRN report of newly joined employees and certify new employees for doing their job as per their job responsibility after completion of departmental TRN.
-
- Quality Head and Plant Head shall be responsible for reviewing and approving the SOP, identifying organizational and occupational TRN needs.
-
- Quality Head and Plant Head shall be responsible for monitoring, reviewing and suggesting any improvement to the schedule/methodology of TRN.
5.0 ABBREVIATIONS USED IN TRAINING SOP:
-
- cGMP: Current Good Manufacturing Practices
-
- TRN: Training
-
- Code: Employee Code
-
- GDP: Good Documentation Practices
-
- GMP: Good Manufacturing Practices
-
- NA: Not Applicable
-
- OJT: On Job TRN
-
- P & A: Personnel and Administration
-
- STC: Site Training Coordinator
6.0 DEFINITION OF WORDS USED IN TRAINING SOP:
-
- Training: A process that involves the acquisition of knowledge, skills, concepts, rules, procedures, processes, etc. Thus, personnel remains competent to perform the task(s) assigned.
-
- Employee: A person who is under the payroll of the organization and is responsible for performing any cGMP activity(s).
-
- This also includes operators and workers who are under the payroll of the organization and are directly responsible for cGMP activities.
-
- Personnel working under the contractual agreement and directly performing cGMP activities shall be considered as employees for TRN purposes.
-
-
Transferred Employee:
-
-
- A person transferred from other Dept. at the same site or same/ other Dept. of different locations.
-
- Copy of TRN record of an employee transferred from one location to another location shall be provided to the TRN Coordinator / Dept. Head of the new location. For TRN purpose such employees shall be treated as a new employee.
-
- Trainee: The person who is being trained on a specific subject or activity.
-
- Trainer / Faculty: One (internal/external) who is a professional with sufficient knowledge, experience (SME) to offer advice and guidance on specific subject or activity by meeting the applicable standards, regulatory and organizational requirements.
-
- Employee Training record: It is a record of TRN activities that the employee has undergone.
-
- Training calendar: It is an Annual TRN Plan covering the TRN needs of individuals or groups.
-
- TRN calendar can be categorized as a central training Calendar and department training calendar.
-
-
Induction training:
-
-
- Training of an employee to introduce/understand the organization systems and policies is termed as Induction TRN.
-
- A TRN which includes familiarization of general concepts like historical background, organizational structure, rules & regulations, the basic concept of cGMP, EHS, facilities, introduction with personnel in various departments, etc.
-
- Functional Training: TRN which is given to the employee after completion of induction TRN at the time of joining the organization or during the Job rotation or assigning a new task.
-
- This training shall include SOP training and Technical skills training.
-
- Phase-I training comprises of Theoretical TRN where trainee reads and understands the desired SOPs. Phase-I training is evaluated by the Questionnaire filled by the trainee.
-
- Phase-II training comprises Practical TRN where the trainee will observe and understand the operation or activity performed by Trainer.
-
- And then trainee performs the activity under the observation of trainer or designee.
-
- Evaluation and certification shall be made by Task champion / Training Coordinator based on the performance of Trainee.
-
- Evaluation of Phase-II shall be done by oral questioning, questionnaire, trainee performance or comparison of results.
-
-
Task Champion:
-
-
- An employee having domain expertise with hands-on experience in performing the particular task is termed as Task Champion.
-
- Task champions are necessarily not required to be a certified trainer.
-
- On the Job Training (OJT): TRN conducted at the shop floor where that particular activity is been done shall be termed as OJT.
-
- Classroom training: TRN conducted at designated places such as a training hall or conference room. In short, any training other than On the Job training is termed as Classroom TRN.
-
- External Training: Any TRN / seminar/conference, which is conducted outside the location, can be termed as external TRN. Any TRN which is conducted by an external faculty ) is termed as External training.
-
- Visitor: A person who is not a group employee and comes for a visit to the location(s) for the purpose of Business (including Services) / Inspection / Audit / Industrial tour etc. is termed as Visitor.
-
- Need-Based Training: TRN has given based on Performance, gap analysis, audit points, Recommendation of Event/Change Control, Regulatory requirements, etc. is termed as need-based TRN.
-
- The Training Cycle: The TRN cycle is a continuous process, which consists of the following elements:
-
-
Identification of Training Needs:
-
-
- The process by which organizations and individuals systematically investigate current and future TRN requirements in relation to the operating environment.
-
- Training Design: The development of an intervention (TRN, job rotation, etc.) to address the identified training needs.
-
- Delivery of Training Program:- The implementation of the learning intervention at individual, group or organizational levels.
-
- Training Evaluation: Without evaluation, we do not know if it has given the desired result. Important steps of TRN cycle are as explained below:
The Learning Cycle
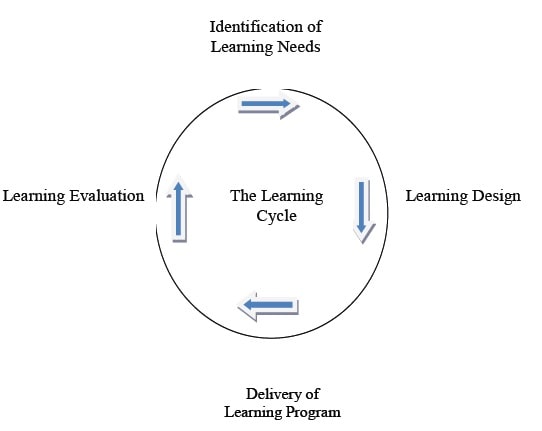
7.0 PROCEDURE FOR TRAINING MANAGEMENT:
-
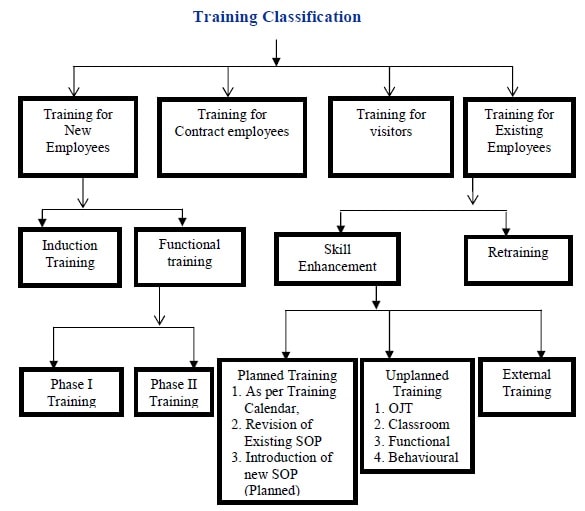
- Training Classification: Training can is broadly classified in the following ways:
-
-
Induction Training (For Newly Joined Employee):
-
-
- Upon newly join of each employee, the Site training coordinator (STC) shall issue the induction TRN schedule and report (Annexure 2).
-
- STC of P&A Dept. shall be responsible for the Coordination of induction TRN for all new joiners (New Staff).
-
- STC shall coordinate with all Dept. HOD for the availability of new staff and shall plan the induction schedule.
-
- After Coordination with Dept. HOD, STC shall attach the SOP matrix with the TRN file.
-
- At the time of recruitment, each employee shall undergo the Induction.
-
- At the time of Induction TRN, Pledge for Ethical Quality Conduct, Individual TRN file, Induction TRN Report (Annexure 2), SOP Matrix and required study materials such as Induction manual shall be given to the new employee by STC.
-
- Original copy of Pledge for ethical quality conduct shall be retained to STC and a signed photocopy of this shall be attached to employee TRN file.
-
- Upon newly join of each employee, STC shall issue the Individual Training File.
-
-
Training file contains:
-
-
- Pledge for Ethical Quality Conduct (Signed Photocopy).
-
- Induction Training Schedule and Report.
-
- Employee Training Summary Sheet.
-
- SOP Matrix.
-
- Training Questionnaire.
-
- Trainer Certificate (If any).
-
- Training Effectiveness Assessment Sheet (If any).
-
- Induction TRN shall be of a minimum of 3 days for all employees.
-
- The purpose of induction TRN is to induct the person for awareness of the following areas such as :
-
-
- Factory Rules and Regulations, Safety Awareness.
-
-
-
- Good Manufacturing Practices.
-
-
-
- Functioning / Systems of various Departments.
-
-
-
- Quality
-
-
- The induction training shall be given by the concerned Dept. Head or his Designee. Concerned Dept. Head or his Designee shall ask questions to the trainee whether he/she has understood the content of TRN.
-
- After successful completion of Induction TRN, the induction report of new join employees shall be checked by STC, reviewed by HOD and approved by Head QA.
-
- After approval, the new joiners shall start the job-specific/functional TRN.
-
-
Job Specific Training :
-
-
- Once Induction TRN is over, new employees shall handover the duly filled Induction TRN Format to Dept. Head after authorization by QA Head,
-
- Which shall be attached to his individual TRN file and undergo departmental training as per the attached SOP matrix (Annexure 4) which is given by STC.
-
- After successful completion of induction TRN. STC shall refer to the SOP matrix for Phase I and Phase II training.
-
- Those SOP list shall be given to the new joinee by STC as per template Annexure 12.
-
- STC shall provide the SOP number, SOP title for easy reference and other details shall be filled by the trainee.
-
-
Phase I Training:
-
-
- The TRN Coordinator/ Trainer (Department) shall introduce the trainee to the department personnel.
-
- Section or Dept. Head / Designee shall decide and define the Job Responsibility (JR) of trainee and approve the JR after completion of the evaluation of Phase I and Phase II training.
-
- Based on SOP Matrix, new joinee shall read departmental SOPs.
-
- SOPs shall be issued by respective Dept. for reading.
-
- In case of any clarity required subject matter expert shall explain the procedure to the new joiner.
-
- The time duration for completion of TRN shall be of a minimum of 3 days.
-
- The trainee shall appear for evaluation through the TRN questionnaire (Annexure 3).
-
- Individual new joinee shall attach an evaluation questionnaire with Annexure 12.
-
- After the successful completion of the evaluation, Dept. Head shall approve the job responsibility of the new employee and prepare the Job responsibility as per SOP.
-
- One photocopy of Job responsibility shall be attached to the TRN file of the employee and a master copy shall be available to STC.
-
-
Phase II Training:
-
-
- Based on successful completion of Phase-I training, the trainee shall be eligible for Phase-II training and the TRN Coordinator /Designee shall prepare the TRN schedule as per JR for Phase-II which is a practical TRN.
-
- Based on SOP Matrix, new joinee shall read departmental SOPs of phase-II.
-
- SOPs shall be issued by respective Dept. for self-reading.
-
- In case of any clarity required subject matter expert shall explain the procedure to the new joiner.
-
- The duration of the Phase-II training can be of 10 days and Trainee shall appear for evaluation through the TRN questionnaire (Annexure 3).
-
- Trainees shall be handed over to Task Champions. Task champion shall explain and demonstrate the activity with Do’s & Don’ts.
-
- The trainee shall observe the activity being performed by the task champion and understand the practical aspect. Depending on the complexity of activity more than one demo is expected.
-
- After satisfactory demo Task champion / Training coordinator shall instruct trainee to perform activity under supervision.
-
-
If any discrepancies or gaps observed Task champion shall explain the same to the trainee.
-
-
- On completion of TRN on one activity, the trainee shall be assigned to the next Task champion for new activity.
-
- Each Task champion shall explain and demonstrate the activity as defined in the procedure.
-
- On satisfactory completion of the entire TRN, the training coordinator shall review the training file and if found satisfactory, forward it to Dept. Head for certification.
-
- Head shall review the trainee’s document and if found satisfactory, shall forward the document to QA Head/designee with the recommendation.
-
- QA Head/designee shall review the trainee’s document and if found satisfactory, approve the recommendation of Dept. Head for completion of Phase-II training.
-
- On satisfactory completion of phase-I and phase-II training trainee now can work independently as per the assigned job responsibility (JR) and TRN file of all individual employee shall be kept to the designated place of their
-
-
Training for Assistant Managers & above:
-
-
- Induction TRN shall be applicable for new Assistant Managers and above, as described in this guideline.
-
- Job-specific TRN at department shall be at least ‘Read-only’ training i.e. Phase-I. However, if a manager or above personnel are intended to perform any activity directly, then he/she shall go through the given procedure of ‘Training for new employees’.
-
- In the case of ‘read-only’, a TRN evaluation shall be required by the questionnaire, which shall be evaluated by TRN coordinator /designee.
-
- Above TRN details shall be recorded appropriately as per attachment-2, attachment-4. All the documents shall be filed along with evaluated questionnaires.
-
- After successful completion of the TRN, he/she shall be eligible to perform the assigned responsibility as per Job Role.
-
-
Evaluation criteria:
-
-
- If the trainee fails to get more than 80% marks:
-
- If the trainee scores less than 80% of total marks, then the trainee shall undergo retraining by training coordinator () The trainee shall appear for evaluation for retraining on the same TRN questionnaire. This shall be done for only one time.
-
- After that the trainee also fails, then TRN Coordinator shall discuss with the Head/Section-in charge and QA Head to decide the further course of action.
-
- If the trainee gets more than 80% marks:
-
- The TRN Coordinator /designee shall discuss the correct answers with those Trainees who shall score more than 80 %, but less than 100 %.
-
- The trainee shall be approved to do his/her specific job as per Job responsibility.
-
- Training Questionnaire Criteria:
-
- A TRN question paper contains a minimum of 5 questions.
-
- Revision of existing SOP/introduction of new SOP, Questionnaire shall be prepared and submitted to QA.
-
- Questions shall be shared in computer drive (K) with limited access
-
- A working copy stamp is not required in the Questionnaire format (Annexure 3).
-
-
Training needs identification:
-
-
- Levels of training need identification:
-
- Organizational need.
-
- Team / departmental Need.
-
- Occupational Needs.
-
- Personal Needs.
-
-
Identification of Training Need:
-
-
- Organizational and Occupational TRN needs shall be identified by Plant Head, Quality Head in consultation with HR Head and STC.
-
- Team / departmental TRN need shall be identified by Dept. Head and training coordinator (Department).
-
- TRN Need identification can be conducted at a variety of levels within the organization
-
- TRN needs identification shall be done through shop floor interaction with the personnel involved.
-
- In the routine activities through a questionnaire.
-
- Based on Event investigation/ Market Complaint /QMS trend / OOS (Out of specification) investigation.
-
- Based on the Shop floor observation trend.
-
- TRN needs identification shall be done while assigning new activity to any person.
-
- In case of regulatory updates like pharmacopeia revision, regulatory guideline updation, etc.
-
- The launch of new product/ technology or in case of procurement of new equipment/ machine
-
- In case of employee repeatedly doing a mistake or poor performance.
-
- In case of the launch of new software is installed, new software users shall be trained by the authorized trainer on software before granting access.
-
-
Assessment Effectiveness of TRN:
-
-
- Each participant’s learning shall be measured by quantitative means.
-
- Wherever evaluation through the questionnaire is not applicable, there shall be a qualitative evaluation done by oral questioning/discussion, etc.
-
- A post-test should be administered so that any learning can be attributed to the TRN program.
-
- TRN effectiveness assessment shall be monitored as per the TRN effectiveness assessment sheet.
-
- This effectiveness sheet shall be applicable for TRN imparted based on event investigation /market complaint investigation / OOS trend /QMS Trend/Shop floor observation trend.
-
- TRN effectiveness shall be evaluated through,
-
-
- Monitoring the particular activity on the shop floor for which TRN is given.
-
-
-
- Discussion with the trainee.
-
-
-
- Oral/written question answer.
-
-
-
- Ensuring communication down the line people.
-
-
-
- Document review.
-
-
- Data review which may include,
-
- Review of event /market complaint / OOS trend/QMS Trend/Shop floor observation trend for the reduced repetitive types of mistakes.
-
- Review of performance records.
-
- Evaluation of On the Job training:
-
- As per evaluation criteria.
-
-
Evaluation of External training:
-
-
- After the TRN Program, meet with course participants to review,
-
- What were the most valuable learnings from this program?
-
- What will you do differently now at work? In which situations?
-
- When will you begin or try this new approach?
-
- What suggestions do you have to improve or customize the course?
-
- Who else should attend this particular TRN program?
-
- Discuss organizational improvement based on the participants’ new learning. Be willing to implement new suggestions on a trial basis with participants involved in tracking and implementation.
-
-
SOP Matrix:
-
-
- Matrix shall be prepared on the basis of TRN need of a new employee in the excel sheet as per Annexure 4.
-
- SOP Matrix shall be prepared based on the experience i.e. Less than 3 years and More than 3 years.
-
- Matrix shall be prepared and updated by STC of the P&A Dept. as and when required. Authorization of SOP Matrix (Soft Copy and Hard Copy) shall be only limited to STC.
-
- On the basis of need, STC shall put (√) on the required SOP. Print out of this shall be given by STC to the employee along with sign and date by STC.
-
- SOP Matrix shall be updated as and when required.
-
-
On Job Training :
-
-
- TRN conducted at the shop floor where that particular activity is been done shall be termed as OJT.
-
-
Classroom training:
-
-
- TRN conducted at designated places such as TRN hall or conference room. In short any TRN other than On the Job training is termed as Classroom TRN.
-
- Any TRN conference/web / telephonic, which is conducted by the same company employee via conference/web / telephonic, trainer signature is not required on the TRN attendance sheet.
-
-
External Training:
-
-
- Any TRN / seminar/conference/web / telephonic, which is conducted outside the location, can be termed as external TRN.
-
- Any TRN which is conducted by an external faculty (not the same company employee) is termed as External training.
-
- The trainer’s signature is not required on the TRN attendance sheet.
-
- After attending the TRN, Trainee shall update the employee TRN summary sheet.
-
- STC shall coordinate for external TRN.
-
-
Retraining:
-
-
- Retraining to be given in the following situations, but not limited to:
-
- If the employee does not have worked for more than 1 year on the techniques/ section for which he was qualified then the trainee shall be re-trained for the techniques/ section before allocating work in the same section.
-
- If an employee is assigned to new Job Responsibility/new work/new area.
-
- Based on Internal/ External audit findings.
-
- Document review observations.
-
- Based on the Shop floor observation trend.
-
-
Unplanned Training:
-
-
- Unplanned TRN shall be imparted in the following situations but not limited to.
-
- Updates of cGMP / Regulatory guidelines.
-
- New or updates of Pharmacopoeia.
-
- Any outcome of Event / OOS/ Product Recall/ Market complaint investigation or any other noncompliance or observation trends.
-
- Any unplanned refresher TRN.
-
- Shop floor/ CAPA base /Need Base like for launch of new software, TRN shall be evaluated based on discussion/ viva-voice/ verbal question.
-
- Such details of discussion/ viva-voice/ verbal questions shall be documented.
-
- In the case of new software, software access shall be given to the person only upon the successful completion of TRN.
-
-
Training for Transferred Employee:
-
-
- Inter department transfer :
-
- Employee’s previous TRN file shall be used to understand the background of individual by concerned department head along with TRN coordinator/Designee to identify the TRN needs.
-
- Training Coordinator/designee shall coordinate the TRN as per procedure under (Job-specific training procedure).
-
- Employee’s previous TRN file shall be retained along with the new TRN record.
-
- Inter location transfer:
-
- In case a person transferred from another location, STC shall ensure that copy of the TRN file of the transferred employee shall be provided to the STC of the transferred location.
-
- STC shall coordinate further TRN as per procedure defined for new employees.
-
- The employee’s previous TRN file shall be retained along with the new TRN file.
-
-
Procedure for issuance of Training Summary Sheet:
-
-
- TRN summary sheet issuance register shall be updated by Dept. Training Coordinator for the issued TRN summary sheet. (Annexure-7).
-
- TRN summary sheet number shall be assigned as per the following system:
-
-
- TSS/DC/YY-XXXX
-
-
-
- Where,
-
-
-
- TSS: TRN summary sheet
-
-
-
- DC: Dept. Code
-
-
-
- YY: Last two digits of the current year
-
-
-
- XXXX: Representing sequential number like 0001, 0002.
-
-
- Department Code
| Department | Code |
| Bonded Store Room | BR |
| Capsulation | CP |
| Coating | CO |
| Compression | CP |
| Environment Health and Safety | ES |
| Granulation | GN |
| Information Technology | IT |
| Maintenance | MN |
| Packing | PK |
| Packing Material Store | PM |
| Personnel and Administration | PA |
| Quality Assurance | QA |
| Quality Control | QC |
| Raw Material Store | RM |
| Sex Hormone | SH |
-
- After logging number in the register, the TRN summary sheet shall be issued by the Department training coordinator.
-
-
TRN number, TRN Allocation Log, and TRN Attendance Sheet :
-
-
- Individual Department allocates their TRN number and maintains TRN number allocation log.
-
- The individual department shall maintain their Individual TRN number.
-
- TRN number shall be assigned to each TRN as per the following system:
-
-
- TRN/DC/YY/XXX
-
-
-
- Where,
-
-
-
- TRN: Training
-
-
-
- DC: Dept. Code
-
-
-
- YY: The last two digits of the current year.
-
-
-
- XXX: Sequential TRN Number like 001,002.
-
-
- After login number in TRN Number Allocation Log (Annexure 9), TRN shall be conducted.
-
- Please explain if TRN is canceled and give a justification in the remark column of Annexure 9.
-
- If TRN is rescheduled beyond one working day a new refreshed TRN number shall allocate.
-
- TRN allocation log shall be issued as PDF in landscape orientation by QA to other departments. Training Coordinator (Department) shall issue PDF copy to their department.
-
- TRN allocation log shall be maintained by the TRN coordinator of the individual department.
-
- After the allocation of training numbers on the attendance sheet, the trainer shall sign on the attendance sheet.
-
- The training attendance sheet shall be issued as PDF by QA to other departments’ training coordinators. TRN Coordinator (Department) shall issue PDF copy to their department.
-
- TRN Effectiveness Assessment Sheet shall be provided to trainees for only Training which shall be imparted on the basis of Review of event /market complaint / OOS trend/QMS Trend/Shop floor observation trend for the reduced repetitive type of mistakes.
-
-
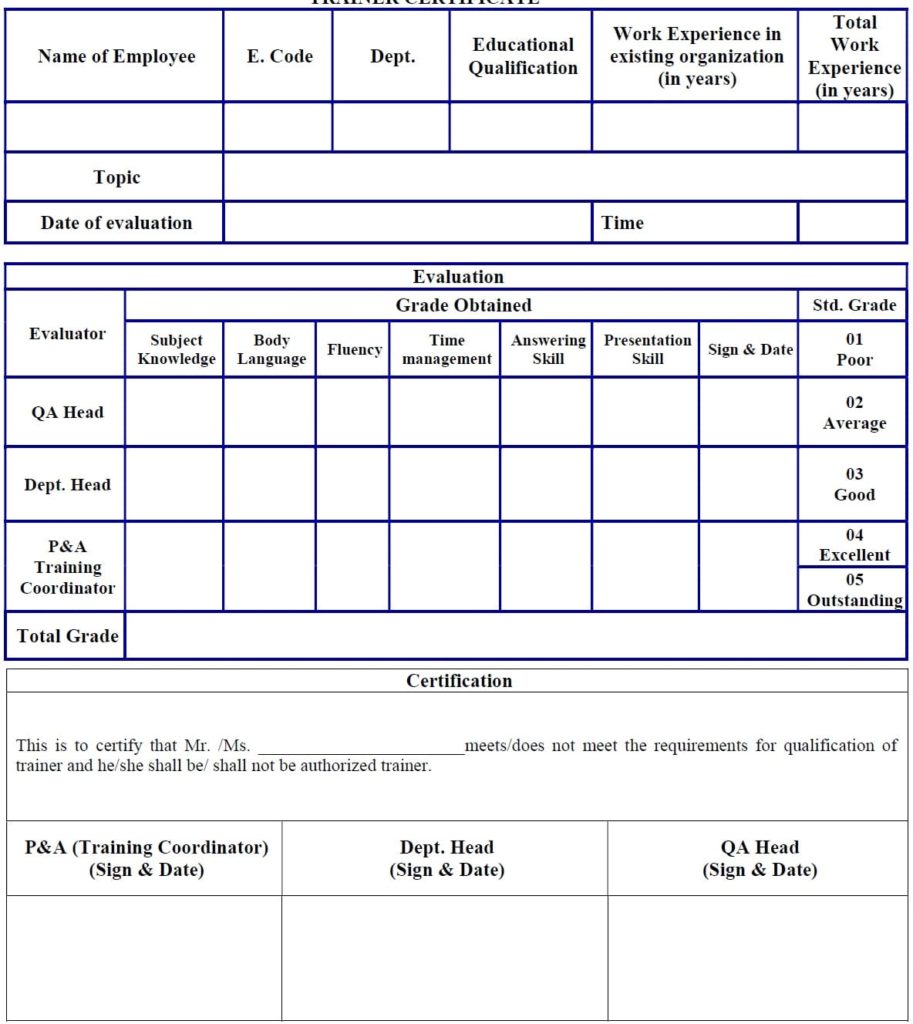
Certification of trainer:
-
-
- The person shall be nominated from each and every department for certification of trainer and they shall be nominated by Department Head.
-
- Any topic for presenting TRN skill shall be given to the nominees by Department Head and QA Head.
-
-
Trainer’s Evaluation criteria:
-
-
- Qualification: Minimum graduate in any faculty.
-
- Experience: Minimum 2 years of working experience with the group.
-
- TRN presentation skill.
-
- After satisfactory evaluation of the trainer, QA Head, Department Head and STC shall certify the trainer (Annexure 10).
-
- The concerned person who shall prepare SOP can be a trainer, but shall not be a certified trainer.
-
- After certification, the trainer list (Annexure-11) shall be updated as and when required by STC.
-
- The assistant manager and above-designated person shall be considered as a certified trainer. There is no need for certification procedures for them.
-
-
Training Calendar:
-
-
- Yearly TRN calendar (planned TRN) shall be prepared (Annexure-6) and maintained by the training coordinator (department).
-
- In the month of December, each department head shall identify the training needs of all the employees working in the department for the next calendar year.
-
- The need identification can be done for an individual or a group of persons having more or less similar job responsibility.
-
- The training calendar shall be of two types.
-
- One shall be Departmental TRN calendar prepared by departmental training coordinator and others shall be Central training calendar which shall be prepared by STC.
-
- Annual training programs shall be structured like cGMP/ ICH/ LIMS/ IT/QMS/HVAC/Water System /Change Control/Event/Market Complaint/OOS and repetitive observations on routine monitoring.
-
- In case of Central Training Calendar, Approval shall be given by Dept. Head, Head QA and P&A Head but in case of Departmental training calendar, Approval from P&A Head is not required.
-
-
Training need identification in Central training calendar shall be defined on the basis of the following considerations, but not limited to
-
-
-
- cGMP
-
-
-
- SOPs
-
-
-
- Safety
-
-
-
- Behavioral
-
-
-
- Developmental
-
-
-
- The central training calendar shall be prepared by STC.
-
-
- One topic shall be decided as a training topic at every month of the training calendar.
-
- The topic shall not be repetitive.
-
- The topics which are applicable for two or more Dept. shall also be covered under the central training calendar, whereas other topics shall be covered under the respective Dept. training calendar.
-
- Training calendar shall be prepared in soft copy after taking annexure in soft from concerned QA .
-
- After completion of preparation, the only soft copy shall be submitted to QA and the user department shall delete it.
-
- A soft copy shall be converted to PDF by QA and then the user department shall use the document as hard.
-
- Approved Copy of Training calendar shall be submitted to QA within the second week of January of New Year.
-
- If training mentioned in the training calendar is not covered in the specific month as per the training calendar, the topic of training shall be covered in the next month and justification shall be provided by the user department and justification shall be approved by the Department head and QA head.
-
- STC, Head QA, Quality Head, and Plant Head shall review and approve the training calendar.
-
-
Retention of Training Records:
-
-
- All Employees shall retain their individual TRN record.
-
- Individual TRN records shall be retained as long as the employee continues to work with the organization.
-
- TRN record of ex-employees of the organization shall be retained with department TRN coordinator/designee for at least 5 years from the date of relieving.
-
- Annual TRN calendar, TRN material shall be retained with Training coordinator/ Designee for a lifetime.
8.0 ANNEXURES:
Annexure 1: Pledge for Ethical Quality Conduct.
-
- Exercise the highest standard of moral and ethical behavior, honesty, objectivity, integrity, and diligence in performance of my duties and responsibilities.
-
- Adhere to the basic philosophy of the GMP system, “Document what you do, and do as per the approved documents”.
-
- Ensure that the data produced by me are scientifically sound, real, authentic, accurately documented and not manipulated.
-
- Share information, observation, and knowledge gained with my colleagues, which may be useful for the investigation and understanding of the root cause of the problems (system and/or product failures) and deriving appropriate Corrective and Preventive Action (CAPA).
-
- Ensure that all company records and documentation regardless of their nature shall be truthfully recorded and maintained in accordance with corporate and regulatory requirements.
-
- They shall be recorded truthfully, promptly, completely and accurately, and shall never distort or disguise the true nature of any action, procedure, or transaction.
-
- Ensure that sign only properly supported documents that I have reason to believe are accurate and truthful.
-
- Accept that purposely false or misleading information related to test results, production records, maintenance records, raw material cards, cleaning logs, calibration records or any other records will not be tolerated and will result in termination and /or other legal action.
-
- There is no exception and no one is allowed to order me or ask me otherwise. Abide and report any violation or apparent violation of this code.
Abide and respond to any future needs of the organization in case of violation of this code even though after leaving the company.
Annexure 2: Induction TRN Schedule and Report.
| Name of Employee | Designation | ||
| Date of Joining | Department |
The Induction Schedule of the new employee for the various departments as under:
| Department/Section | Date and Time | Trainer Name
& Sign |
Trainees Remark
Understood /Not Understood |
Trainees Sign&Date |
| P&A | ||||
| IT | ||||
| EHS | ||||
| Warehouse | ||||
| Granulation | ||||
| Compression | ||||
| Coating/Capsule | ||||
| Packing | ||||
| Maintenance | ||||
| Quality Control | ||||
| Quality Assurance |
|
Department |
Training
done by ( Name) |
Sign and Date |
Personnel and Administration |
||
| Introduction | ||
| A short summary of Company History | ||
| Organization and Personnel policies | ||
| Factory Rule and Regulation | ||
| Entry and Exit procedures. | ||
| Safety precautions | ||
| Personal Hygiene | ||
| Dress Code | ||
| Training Program | ||
| Medical Examination Requirement | ||
| Pest control procedures | ||
| Pledge for ethical quality conduct |
Information Technology |
||
| Introduction of IT Department | ||
| IT current Practices | ||
| ERP and Lotus Notes |
Environment Health & Safety |
||
| Introduction & Safety Policies | ||
| Personal Protective Equipment | ||
| Fire Extinguisher | ||
| Operational Safety, Emergency | ||
| ETP Operation | ||
| Waste Management | ||
| EHS Awareness, Campaign and Reporting |
Warehouse |
||
| Introduction | ||
| Material Management, Identification, and Labeling | ||
| Warehousing Procedure | ||
| Dispensing Procedure of Materials |
Manufacturing (Granulation/ Compression/Coating/Capsule) |
||
| Introduction | ||
| Importance of GMP | ||
| Documentation | ||
| Mfg. Flow (Mfg. Stage) | ||
| Critical Process Control | ||
| Environmental Condition | ||
| Type of Equipment Used with their Working Principle | ||
| Handling of Equipment Breakdown |
Packing |
||
| Introduction | ||
| Type of Equipment Used with their Working Principle | ||
| Packing Operation | ||
| System of Packaging Related | ||
| Flow of Material (Finish Goods) |
Maintenance |
||
| Introduction | ||
| Utilities and Facilities System | ||
| Water System | ||
| HVAC System | ||
| Compressed Air | ||
| Preventive Maintenance System |
Hormone Department |
||
| Introduction | ||
| Dress Code | ||
| Equipment Layout/Usage/Maintenance/Safety | ||
| Documentation | ||
| Critical Process Control | ||
| Building and Facility with respect to Area |
Quality Control |
||
| Introduction | ||
| Quality Control Activities | ||
| RM/PM Sampling and Retesting | ||
| Calibration Program | ||
| Instrument Briefing | ||
| Handling of OOS | ||
| GLP/cGLP and Laboratories Safety |
Quality Assurance |
||
| Documentation | ||
| Change Control System | ||
| Event Reporting and Investigation | ||
| Good Documentation Practices | ||
| Self Inspection | ||
| Schedule M |
Annexure 3: TRN Questionnaire.
| Name of Trainer | Topic | ||
| Department | |||
| Designation | Training No. | ||
| Name of Trainee | Date | ||
| Employee Code | Time Duration | ||
| Department | Total Marks | ||
| Each Question carries marks | Acceptance criteria for passing | Not Less Than 80% |
Q 1.
Ans.
Q 2.
Ans.
Q 3.
Ans.
Q 4.
Ans.
Q 5.
Ans.
| Sign of trainee
(Before Evaluation) |
Evaluation done by
(Trainer/Training Coordinator) sign and date) |
Marks obtained |
Retraining: (Required /Not required).________________
Remarks after evaluation: (Qualification / Not Qualified) _________
Note: 1 – If obtained marks is less than 80%, explain the answer for the incorrect answer.
2 – Attach this answer report to individual training files.
Annexure 4: SOP Matrix.
| SOP No. | SOP Title | Phase | Less than 3 year | More than 3 year | |||||||
| QA | QC | WH | … | … | QA | QC | WH | ||||
| SOP…. 001 | |||||||||||
| 002 | |||||||||||
| 003 | |||||||||||
| 00…n | |||||||||||
Annexure 5: Employee TRN Calendar.
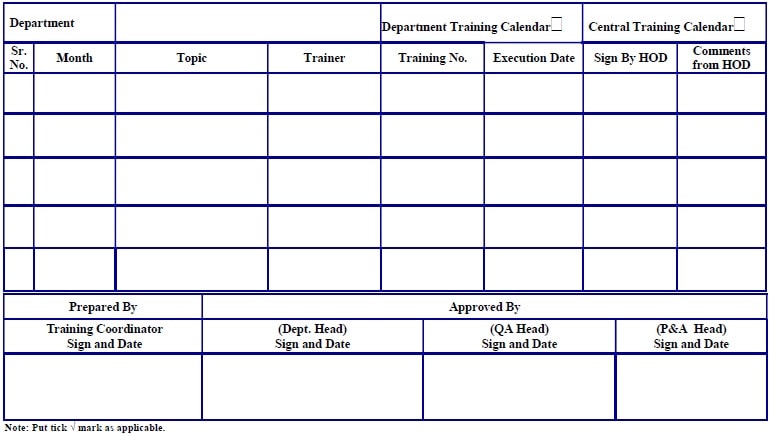
Annexure 6: TRN Attendance Sheet.
| Topic of Training | |||
| Training Number | Date of Training | ||
| Trainer Name | Trainer’s Department | ||
| Trainer’s Designation | Time Duration | ||
| Starting Time | Completion Time | ||
| Venue | |||
| Type of Training: Planned (OJT/Classroom)/ Unplanned (OJT/Classroom)/ Classroom/External (OJT/Classroom)/Retraining (OJT/Classroom) (tick wherever applicable.) | |||
|
Sr. No. |
Trainees Name | E.Code | Department | Trainee’s Remarks
(Understood/ Not Understood) |
Trainee’s Signature |
Annexure 7: Employee TRN Summary Sheet
| Name | Training Summary Sheet No. | ||
| Designation | Employee Code | ||
| Department | Date of Joining |
|
Sr. No. |
Date of Training | Type of Training* | Topic of Training | Training Number | Trainer Name | Duration in Minutes |
Trainee Sign & Date
|
Annexure 8: Employee TRN Summary Sheet Issuance Register
| Date | Name of Employee | E. Code | Department | Employee Training Summary Sheet No.
(TSSN/DC/YY-XXXX) |
Issued by
(Sign & Date) |
Received by (Sign & Date) |
Annexure 9: TRN Number Allocation Log.
| Department | ||||||||
| Date | Topic of Training | SOP No./ Doc. No. | Trainer Name | Type of Training | Training No.
(TRN/DC/YY/XXX) |
Allotted by
Sign & Date |
Remarks | |
Annexure10: Trainer Certificate.
Annexure11: List of Certified Trainer.
Prepare the list of certified trainers with the following table contains…
-
- Sr. No
-
- Name of Employee
-
- E. Code
-
- Department
-
- Competent Area
-
- Qualification
-
- Total Experience
-
- Date of Joining
-
- Remarks
Annexure12: Job-Specific TRN Schedule Template.
| Phase I ( ) Phase II ( ) | ||||||||
| Trainee Name | Employee Code | |||||||
| Department | Date of joining | |||||||
| Mode of Training | Self-reading ( ) Explanation ( ) Classroom ( ) OJT ( ) | |||||||
| SOP No. | SOP Title | Training Date | Trainee
Sign & Date |
Evaluation Status (% of Marks obtained) | ||||
| Enclosure: Training evaluation Questionnaire(s) | |||
| Evaluation done by
(Training Coordinator/Designee) |
Sign & date |
Qualify/ Does not Qualify
(Tick as applicable) |
|
| Certification: Mr./ Ms. ___________________________ has successfully completed the phase-I/ phase II job specific trainings. | |||
| Certified By:
Department Head ( Sign & Date) |
QA Head/designee (Sign & Date) | ||
Annexure13: TRN Effectiveness Assessment Sheet Template
| Topic/SOP: | |
| Reference Document and Document No: | |
| Date of training: | |
| Date of Assessment: | |
| Need of Assessment: | |
| Mode of Training Effectiveness Monitoring | Oral / Written Question-Answer/Monitoring Particular activity/Data and Record Review/Self-Inspection |
| Sr. No. | Name of Participants | Signature & Date |



Pingback: Microbiologist Qualification : SOP & Protocol - Pharma Beginners
Pingback: Self Inspection Checklist & Internal Audit Formats - Guidelines - SOPs
Pingback: Environmental Monitoring (EM) - New Approach Guide - Pharma Beginners
Pingback: Analyst Qualification of Quality Control Personnel - Guidelines - SOPs
Pingback: SOP for Workplace During COVID-19 (Corona Virus) - Pharma Beginners