Standard Operating Procedure (SOP) for Qualification of Instrument, Equipment, Facility & Utility in the pharmaceutical manufacturing plant.
Qualification: The action of proving and documenting that equipment or utility is properly installed, work correctly, and actually produce the expected results consistently.
Procedure for Qualification of Equipment, Instrument, Facility & Utility
1.0 PURPOSE:
-
- The purpose of this SOP is to provide a guideline for carrying out the qualification at pharma plant.
2.0 SCOPE:
-
- This SOP is applicable for the qualification of all equipment, instrument, facility, and utilities at pharmaceutical manufacturing plants.
3.0 REFERENCES:

-
- SOP for Change Control Management.
-
- SOP for Quality Risk Management,
4.0 RESPONSIBILITY:
-
- The user department shall be responsible for-
-
- Preparation of the User Requirement Specification (URS) w.r.t. Equipment, Instruments, Facility and Utility.
-
- Preparation of design qualification (if required).
-
- Qualification protocols preparation.
-
- The user shall notify to Plant Head for Qualification of each & every Equipment, Instrument, Facility, and Utility.
-
- The engineering department shall be responsible for –
-
- Providing technical assistance to the user department for the preparation of required documents.
-
- Installation of machines, utility at the designated place.
-
- QA department shall be responsible for –
-
- Review protocols and provide technical inputs.
-
- Review qualification reports for its completeness and correctness w.r.t. all data and report.
-
- Preparation of Qualification master plan.
-
- All Department Head shall review and sign all protocols, reports, and Qualification master plan.
-
- Site Quality Head shall approve protocols and reports and Qualification master plan.
-
- External Agency shall provide technical assistance for preparation of documents and execution of activities whenever require.
5.0 ABBREVIATIONS (Equipment Qualification SOP):
-
- AHU: Air Handling Unit.
-
- EQMP: Equipment Qualification Master Plan.
-
- FAT : Factory Acceptance Test
-
- FDS : Functional Design Specification
-
- HVAC: Heating, Ventilation & Air Conditioning.
-
- IQMP: Instrument Qualification Master Plan.
-
- LAF: Laminar Air Flow
-
- PAO: Poly Alfa Olefin.
-
- RLAF: Reverse Laminar Air Flow.
-
- SAT: Site Acceptance Test
6.0 DEFINITION:
-
- Equipment Qualification Master Plan (EQMP):
-
- A summary document prepared as part of project planning that describes overall philosophies, approaches, and objectives to all aspects of the qualification.
-
- The document defines responsibilities and expectations for the various steps of the qualification exercise and also establishes timelines for completion of each component.
-
-
Instrument Qualification Master Plan (IQMP):
-
-
- Prepare a summary document as part of planning that describes overall philosophies, approaches, and objectives to all aspects of the qualification.
-
- The document defines responsibilities and expectations for the various steps of the qualification exercise and also establishes timelines for completion of each step.
-
- Critical equipment:
-
- Critical equipment is the equipment needed for processing, packaging, holding, or supports of products that have the potential to direct impact in critical process parameters and quality of the product.
-
-
Non-critical equipment:
-
-
- Non-critical equipment is the equipment needed for processing, packaging, holding, or supports of products that do not have the potential to direct impact in critical process parameters and quality of the product.
-
-
User Requirement Specification (URS):
-
-
- The documented requirement of the equipment, the utility for its intended purpose. Functional design specification (FDS) according to cGMP and regulatory requirements.
-
-
Design Qualification (DQ):
-
-
- Documented verification to ensure that the proposed design of the equipment, utility is suitable for the intended purpose at it meets in all aspects.
-
-
Installation Qualification (IQ):
-
-
- Documented verification that the equipment, utility as installed or modified, comply with the approved design, manufacturer’s recommendations and user requirement. FAT & SAT to be added.
-
-
Operational Qualification (OQ):
-
-
- Documented verification that all the components of a system or of a piece of equipment operate as specified. This involves testing of all normal operating controls; all alarm points, all switches, and displays, interacting controls, and any other indications of operations and functions.
-
-
Performance Qualification (PQ):
-
-
- Documented verification that the equipment, utility is performing effectively and reproducibly, based on approved method and specifications.
-
-
Factory Acceptance Test (FAT):
-
-
- Documented verification to ensure of the compliance of the equipment at vendors site against approved design.
-
-
Site Acceptance Test (SAT):
-
-
- Documented verification to ensure of the compliance of the equipment at user site against approved design as well as against FAT.
-
-
Re-qualification:
-
-
- Reconfirming that area, equipment, instrument, utility, the system is meeting the predefined acceptance criteria after major changes in the parameters or after a definite period, if there is no major change.
Related: SOP for Laboratory Instrument Qualification
7.0 PROCEDURE FOR EQUIPMENT QUALIFICATION:
-
-
Preparation of Qualification master plan:
-
-
- Equipment Qualification Master Plan is an umbrella document that provides an overall philosophy, intention and methodology to be adopted for qualification.
-
-
Equipment Qualification Master Plan:
-
-
- EQMP-Equipment Qualification Master Plan is designed to demonstrate the approach for the qualification of equipment, Utility to meet the current National or International regulatory guidelines.
-
- Equipment Qualification Master Plan is designed to provide guidelines for planning, execution and successful completion of the equipment/utility qualification.
-
- The intent of this qualification master plan is to provide a written plan for establishing documented evidence of the suitability of facilities and consistency of equipment/ utilities to reproduce the desired results.
-
- Following is the numbering system of the Equipment Qualification Master Plan.
BS/EQMP/XX
where,
BS is Location code
EQMP stands for Equipments Qualification Master Plan
XX is version no. start from 00
-
-
Equipment Qualification Master Plan shall include below-mentioned details, but not limited to,
-
-
-
- Introduction and Philosophy,
-
-
-
- Scope and reason for Revision,
-
-
-
- Responsibility,
-
-
-
- References and Annexure,
-
-
-
- Definition,
-
-
-
- Qualification approach,
-
-
-
- User Requirement Specification (URS),
-
-
-
- Design Qualification (DQ),
-
-
-
- Factory Acceptance Test (FAT),
-
-
-
- Site Acceptance Test (SAT),
-
-
-
- Training Program,
-
-
-
- Installation Qualification (IQ),
-
-
-
- Operational Qualification (OQ),
-
-
-
- Performance Qualification (PQ),
-
-
-
- Qualification protocol detail,
-
-
-
- Provision for Investigation in case of non-conforming results / Deviation,
-
-
-
- Planned Preventive Maintenance Program,
-
-
-
- Requalification criteria,
-
-
-
- Supplement provision,
-
-
-
- Critical equipment/ utility Performance Qualification procedure and acceptance criteria
-
-
- QA shall prepare Equipment Qualification Master Plan. Production Head, QA and Maintenance Head shall review.
-
- QA Head shall approve the Equipment Qualification Master Plan.
-
- After approval and training, QA shall mention the effective date on the first page.
-
- Specimen of Header and Footer of Equipment Qualification Master Plan is as per Annexure-1.
-
-
Instrument Qualification Master Plan
-
-
- This Instrument qualification master plan is designed to demonstrate the approach for qualification to meet the current National and International regulatory guidelines.
-
- Following is the numbering system of Equipment Qualification Master Plan
BS/IQMP/XX
where,
BS stands for location code.
IQMP stands for Instrument Qualification Master Plan
XX is a numerical serial no. start from 00
-
- Whenever any revision in IQMP the numerical serial no. shall be changed from 00 to 01.
Related: SOP for Laboratory Instrument Qualification
-
- QC shall prepare Instrument Qualification Master Plan. and reviewed by QA & Head QC.
-
- Site QA Head shall approve the IQMP. After approval QC shall mention the effective date on the first page.
-
- Specimen of Header and Footer of IQMP is as per Annexure-2.
-
- Head QA or designee shall maintain the original copy of all master plan.
-
-
Revision of Qualification master plan – Equipment Qualification
-
-
- Revise all qualification master plan after every two years unless otherwise there is a need for revision arising out of any review, any audit finding or to incorporate any new requirement.
-
-
Qualification Methodology – Equipment Qualification
-
-
- Facilities, Utilities, and Equipment used for manufacturing, processing, packaging, labeling, storing, testing and controlling of drug products Shall be qualified prior to use.
-
- Perform the Qualification for new equipment/ Instrument/ utility/ facility, after major breakdown in equipment/ utility, after modification in equipment/ Instrument/ utility and facility.
-
-
Qualification for introduction of a new equipment/ facility/ utility
-
-
- After receiving the new equipment/ facility/ utility user department shall take the change control for its qualification and QA shall update the Equipment Master List if this change control is in case of equipment.
-
- Assign the Equipment no. as per the SOP for Equipment Numbering System.
-
- Maintain the master lists of critical and non-critical equipment in soft copy as per annexure 6 & 7 respectively, and revise as and when required.
-
- Execute the following documents for new equipment/ facility/ utility following documentation to demonstrate the conformance of equipment to design, characteristics, and capabilities specified in required documents.
-
-
- User requirement specification (URS)
-
-
-
- Design qualification (DQ)
-
-
-
- Factory Acceptance Tests (FAT)
-
-
-
- Site Acceptance Test (SAT)
-
-
-
- Installation Qualification (IQ)
-
-
-
- Operation qualification (OQ)
-
-
-
- Performance Qualification (PQ)
-
-
-
User Requirement Specification- Equipment Qualification
-
-
- The URS is made to verify that the owner/ user requirements, which include the establishment of critical operating or operational parameters or specifications before the final design agreed, have been met.
-
- The user shall prepare the URS considering all operational, safety and GMP requirements.
-
- The user requirement shall submit to the manufacturer/ supplier, based on which manufacturer/ supplier will prepare the design.
-
- Change Control Management:
-
- The user department shall initiate a Change Control as per SOP for Change Control Management only after confirmation of Purchase order.
-
- After approval of Change Control, Initiate the preparation of the respective protocol.
-
-
Design Qualification: Equipment Qualification
-
-
- The DQ is made to verify that the owner/ user requirement, which includes the establishment of critical operating or operational parameters or specifications before the final design is agreed, has been met.
-
- Based on URS, the manufacturer/ supplier shall prepare design qualification documents and submit to the user for approval.
-
- On the basis of approved design qualification documents, the manufacturer/ supplier shall start manufacturing/ fabricating the equipment/ utility.
-
-
Factory Acceptance Test (FAT): Equipment Qualification
-
-
- The FAT is prepared to verify that the main items or system meets design specifications and conforms to agreed performance intent.
-
- The user shall prepare FAT protocol according to URS/ DQ, manufacturer specification and purchase order.
-
- Ensure that the equipment/ system is manufactured as per designed specification at the manufacturers’ site.
-
- The user shall also check the basic performance of the equipment/ system delivered at the plant meets the design specification.
-
- User shall execute approved FAT protocol at the manufacturer’s site with QA and Engineering representative.
-
- The entire test shall be performed and reported by the supplier. All tests performed during FAT must be performed in accordance with reviewed and approved protocol and procedure in the presence of the user.
-
-
Site Acceptance Test (SAT): Equipment Qualification
-
-
- The SAT is to establish documented evidence that the receipt of the equipment at the site confirms with the standards laid down in the protocol, FAT, purchase order and manufacturer’s specification.
-
- User shall prepare SAT protocol according to the manufacturer specification, purchase order and FAT report.
-
- User shall execute approved SAT protocol and will check for all tests mentioned in protocol with the QA & maintenance representative at the site when item/ equipment/ system reaches the factory premises and reported by the production and engineer.
-
-
Installation Qualification : – Equipment
-
-
- Carry out the Installation Qualification of Equipment/ utility to ensure that the installed equipment/ utility according to design documents, purchase. Specifications, FAT and SAT report& change control.
-
- The parts of the systems, which are dismantled prior to shipping, shall be noted and be verified again after re-assembly at the final site during Installation Qualification.
-
- Inspect Equipment/ utility either visually or by measurement for its critical parts. Wherever applicable other instruments shall be used for qualification purposes.
-
- Check all the relevant tests mentioned in the protocol after the proper installation of equipment.
-
- Check the calibration certificate of the instrument attached to equipment and other related formats before starting operation qualification.
-
- After completion of IQ, Release the equipment shall be released for OQ.
-
-
Operation Qualification :
-
-
- The Operational Qualification is carried out to verify that an Equipment/ system or sub-system performs as intended throughout all anticipated operating ranges.
-
- Operation qualification activities shall be started only after completion of successful installation qualification.
-
- QA and User representative shall execute the approved protocol, which is used earlier during installation qualification.
-
- The user department shall verify proper operation by performing the critical operating parameters that have a significant impact on the equipment able to operate and meet specifications satisfactory.
-
- User department shall prepare final conclusion after the test functions are checked and observed within specification.
-
- After completion of OQ, the equipment shall be released either for PQ or for routine use as the case may be.
-
-
Performance qualification:
-
-
- The performance qualification is carried out to provide documented evidence that an integrated system or processing operation is capable of performing consistently (during multiple cycles or extended periods) to give an outcome that meets predetermined specifications.
-
- After successful completion of equipment Operational Qualification, all equipment shall be subjected to performance qualification prior to use.
-
- The extend of Performance qualification activity may vary to the principle of operation (make/ model/ type of equipment/ type of material/ product to be processed.)
-
- For Example, the performance qualification of RMG will differ from than Blister packing machine, the Compression machine will differ from FBD, etc.
-
- In case the functionality or performance is dependent on the material/ product characteristics (like granules property: size, flow, cohesiveness, tablet hardness, thickness etc.), then equipment shall be qualified for routine use with respect to the product/ materials to be used.
-
-
Therefor performance qualification of equipment like RMG, FBD, Compression machine, coating machine etc. to be qualified with the product during process qualification.
-
-
- If the product characteristic is not dependent on the performance of the equipment, then PQ shall be done with any three consecutive batches (eg. Equipment like leak test apparatus, induction sealing machine, blister packing machine, etc.)
-
- PQ can be performed on commercial/ Placebo/ Dummy batches for trials of new equipment. If commercial batches were then batches shall be released only after completion of qualification of the equipment.
-
- PQ shall be performed for one batch with optimum load in case of schedule re-qualification.
-
- After completion of execution, all raw data and reports shall be compiled and a final conclusion shall be drawn.
-
- The final report shall be prepared, summarizing the results obtained, commenting on any deviation observed and handled through proper justification.
-
- After final approval of the conclusion/ report by Site Quality Head the respective equipment, instrument, facility, and utility shall be allowed for routine use.
-
- After the final approval of the report. Provide the Handover Certificate of Equipment as per Annexure-8.
Note: Operation and Performance Qualification shall be carried out only if desired utility is available and environmental conditions (wherever applicable) are achieved in the area and same shall be recorded in the Qualification protocol.
-
-
Qualification for the introduction of a new Instrument.
-
-
- For new Instrument following activities shall be done to demonstrate conformance to design documents, characteristics, and capabilities specified in requirements documents.
-
-
- Installation Qualification (IQ)
-
-
-
- Operation qualification (OQ)
-
-
-
- Performance Qualification (PQ)
-
-
- Installation Qualification (IQ) : Carry out IQ for new Instrument as per the procedure mentioned above.
-
- Operation qualification (OQ) : Carry out OQ for new Instrument as per the procedure mentioned above.
-
- Performance Qualification (PQ) : Carry out PQ for new Instrument as per the procedure mentioned above.
-
-
Protocol Preparation for FAT, SAT, IQ, OQ, and PQ
-
-
- The protocol for Qualification (FAT/ SAT/ IQ / OQ / PQ) shall address and include, but not necessarily be limited, to the following topics.
-
- FAT : Approval, purpose, procedure, verification criteria, Manufacturer’s Machine Identification Code / Identification No., Final report approval.
-
- SAT : Approval, purpose, procedure, Documentation, Verification criteria, Final report approval
-
- IQ/OQ/PQ: Purpose, Scope, Responsibility, Intended Use, Location, Reference, History, Attachments, Study for qualification, Responsibilities, Signature log, Training record
-
- IQ: Equipment/ Instrument Detail, Procedure, Installation qualification table, Conclusion
-
- OQ: SOP training, Procedure, Operational qualification table, Observed deviation, Conclusion
-
- PQ: Procedure, Acceptance criteria, Observed deviation, Final Conclusion
-
- Report approval sheet
-
- Specimen of Header and Footer for above protocol is as per annexure-3. After approval QA shall mention the effective date on first page.
-
- The numbering and Issuance system for Qualification protocol shall be as per SOP for Protocol Numbering and Issuance System.
-
- If Vendor’s Qualification Protocol complies and meets the requirements as per Organization Standards, that protocol can be used for execution of the qualification. For this user department should have to take prior approval as per Annexure-10.
-
-
Area Qualification :
-
-
- Area Qualification is carried out to provide the documentary evidence that a particular area is constructed and qualified as per predefined specifications.
-
- Perform the below-mentioned activities during the execution of Area Qualification, but not limited to.
-
-
- Construction and finishing of wall, floor, doors, view panels and ceiling.
-
-
-
- Dimension measurement wherever applicable.
-
-
-
- All the required utilities and other facilities supplied.
-
-
-
- Area Layout/ drawing, Room identification & location.
-
-
-
- Lighting Lux of every room in all four corners and center of the room.
-
-
-
- Temperature Mapping for Warehouse/ Quarantines/ Recovery room.
-
-
-
- Environmental condition monitoring for the classified areas.
-
-
-
- Water drainage ability of drains.
-
-
-
- Cleaning and Sensitization Procedure.
-
-
- User department shall prepare the qualification protocol and organize the qualification study in co-ordination with Quality Assurance and Engineering department.
-
- Protocol/ Report shall be finally reviewed by QA and approved by QA Head &Site Quality Head.
-
- After final approval of the report Area, the Handover Certificate shall be provided as per Annexure-9.
-
-
Qualification of AHU system:
-
-
- HVAC qualification shall be carried out to supply the required air quality to the various section of the individual departments, to provide product protection from airborne contamination, to maintain the temperature and humidity, to provide differential room pressure or airflow movement and to provide product protection from cross-contamination.
-
- Engineering department shall prepare the qualification protocol and organize the qualification study in co-ordination with Quality Assurance.
-
- Engineering person shall record the observations as per designed protocol and prepare a report.
-
- All AHU’s like HVAC system shall be qualified as per procedure described in SOP-BSMN/030-“Area Qualification” against approved protocol.
-
-
Microbial Count:
-
-
- Environmental monitoring of the area shall be executed using a settling plate as well as RODAC plate respectively by exposing the settle plate at the pre-specified location as per sampling plan, maintained by microbiology department (Reference SOP: BSQM/033-Environmental Monitoring in Manufacturing Area.)
-
- Protocol/ Report shall be finally reviewed by QA and approved by QA Head & Site Quality Head.
-
-
Qualification of LAF & RLAF :
-
-
- Qualification of LAF & RLAF shall be carried out to provide the air with high-pressure compare to the surrounding area and to prevent microbial and particulate matter contamination during dispensing/sampling of Raw material, prevent dusting during dispensing/ Sampling.
-
- All LAF and RLAF shall be qualified as per procedures described in point no. 7.4 and 7.5 as per approved protocol.
-
- The user department shall prepare the qualification protocol and organize the qualification study in co-ordination with QA & Engineering department.
-
- The numbering and Issuance system for Qualification protocol shall be as per SOP no. BSQA/010.
-
- Installation Qualification: IQ of LAF & RLAF shall be carried out to check LAF/ RLAF size as per requirement, check pre-filter, motor blower, Magnehelic gauge, and final HEPA filter’s test certificates as per specification.
-
- Operational Qualification: Carry out OQ of LAF/ RLAF to run the LAF/ RLAF and to check the operational parameter functioning
-
- Performance Qualification: Carry out PQ of LAF/ RLAF to check LAF/ RLAF air velocity, Airflow pattern, air cleanliness by non-viable particle count and HEPA filter efficiency & integrity, etc.
-
- The user department shall record the observations as per the designed protocol and prepared a report.
-
- QA shall review the Protocol/ Report and approved by QA Head & Site Quality Head.
-
-
Qualification of Water System :
-
-
- Carry out the water system qualification to generate Potable water & purified water of desired quality.
-
- The engineering department shall prepare the qualification protocol and organize the qualification study in coordination with QA.
-
- Qualification protocol shall carry the following details but not limited to:
-
- Installation Qualification: Equipment/instrument details, procedure, Acceptance criteria, Prequalification, Installation check, summary & conclusion.
-
- Operational Qualification: Training, Procedure, Acceptance criteria, Operating inputs, summary & conclusion.
-
- Performance Qualification: Study plan, sampling Frequency, user point, summary & report.
-
-
Carry out the performance qualification of the water system in three phases.
-
-
- In phase 1, the water quality parameter shall be evaluated for 14 days, during this phase of validation water can not be used for manufacturing purposes. After successful evaluation of water quality, proceed for next phase of water validation.
-
- In phase 2, the water quality parameter shall be analyze for 14 days, during this period water can be used for manufacturing purposes.
-
- In phase 3, the water quality parameter shall be analyzed for one year according to the routine sampling plan to evaluate the impact of seasonal changes on the quality of water.
-
- Maintenance department & Microbiology department shall record the observations as per designed protocol and prepared a report.
-
- Protocol/ Report shall be finally reviewed by QA and approved by QA Head &Site Quality Head.
-
- Numbering and Issuance system for Qualification protocol shall be as per SOP no. BSQA/010.
-
-
Re-qualification strategy:
-
-
- Equipment / utility shall re-qualify either in the following conditions.
-
-
- Major Break Down
-
-
-
- After modification in equipment, utility, facility which may have an impact on product quality only.
-
-
-
- The design change of spares that have an impact on the performance of equipment and quality of the product.
-
-
-
- In case of during Location change of equipment. (In case of balances only recalibration).
-
-
-
- As per scheduled re-qualification of critical and non-critical equipment/ utility.
-
-
- OQ & PQ shall be performed during re-qualification. Perform PQ with one batch during re-qualification.
-
-
Re-qualification criteria for critical equipment
-
-
- Perform the operational and performance qualification as per approved protocol for the re-qualification of critical equipment.
-
- Re-qualify all critical equipment in every 5 years ± 3 months.
-
- Perform only OQ and PQ (if required) during the re-qualification of critical equipment/ utility.
-
- Conduct PQ with one commercial/ Dummy/ Placebo batch only.
-
-
Re-qualification criteria for non-critical equipment.
-
-
- Conduct the re-qualification of non-critical equipment whether there is a significant change that has an influence on the quality of the product.
-
- For re-qualification of non-critical equipment, history of maintenance and utilization of the equipment shall be reviewed and documented as per format of Requalification of non-critical Equipment (Annexure-4)
-
- Qualification of all non– critical equipment. every 5 years ± 6 months.
-
-
Re-qualification criteria for Instrument in the following conditions.
-
-
- When the instrument is shifted from one location (Change in premises) to another location, take Permanent change control re-qualification (IQ, OQ, and PQ) shall be required.
-
- The instrument is upgraded or after having a major repairing, qualification (OQ/ PQ) required.
-
- When the instrument is shifted within the laboratory premises (one room to another room), requalification shall not be required, in house, calibration shall be required.
-
-
Re-qualification criteria for AHU System
-
-
- Carry out re qualification of AHU (HVAC) in below mention criteria, but not limited to
-
-
- The major change in equipment, Change of spare/ parts that have a direct impact on the Performance of the equipment.
-
-
-
- Schedule re-qualification.
-
-
- The frequency of different tests for the Re-qualification of AHU shall be as per the below table as suggested in ISO-14644.
| S. No. | Test Parameter | Maximum Time Interval |
| 1. | Airborne Particle Count (≤ISO Class 5) | 6 Month ±10 Days |
| 2. | Airborne Particle Count (>ISO Class 5) | 12 Months ± 30 Days |
| 3. | Air Flow Velocity Test | 12 Months ± 30 Days |
| 4. | Installed Filter Leakage Test | 12 Months ± 30 Days |
| 5. | Airflow visualization Test | 24 Months ± 60 Days |
| 6. | Temperature Test | Commissioning/ Daily |
| 7. | Humidity Test | Commissioning/ Daily |
| 8. | Recovery Test | 24 Months ± 60 Days |
| 9. | Containment Leakage Test | Commissioning |
| 10. | Air Pressure Difference Test | Commissioning/ Daily |
-
-
Re-qualification criteria for LAF & RLAF.
-
-
- Re qualification of LAF & RLAF shall be carried out in below mention criteria, but not limited to.
-
-
- Change in the location of the equipment/ system.
-
-
-
- The major change in equipment, Change of spare/ parts that have a direct bearing on the Performance of the equipment.
-
-
-
- Critical gauges shall be replaced or corrected if the gauge is found out of calibration during the calibration of the gauges.
-
-
-
- Schedule Requalification.
-
-
- Frequency of Re-Qualification of LAF & RLAF shall 6 months ± 10 days, for Airborne Particle Count, frequencies for remaining tests shall be the same as for AHU mentioned above.
-
-
Re-qualification criteria for utilities
-
-
- Carry out the re-qualification to ensure that change/ modification in utilities remains under control and within the parameters defined and certified.
-
- Carry out the re-qualification of utilities in below mention criteria, but not limited to.
-
-
- Change in location.
-
-
-
- Any modification that has the potential to impact product quality.
-
-
- Re-Qualification of Compressed Air.
-
- Carry out the re-qualification of Compressed Air against parameters mentioned in SOP but not limited to parameters mentioned in the concerned SOP.
-
-
- In case of any modification impacting the product quality.
-
-
-
- Scheduled re-qualification after every 1 year ± 1 month.
-
8.0 ANNEXURES:
-
- Annexure 1: Header & Footer specimen of Equipment Qualification Master Plan (EQMP)
| Title: EQUIPMENT QUALIFICATION MASTER PLAN |
Page x of y |
|
| Document No.: |
CCR No. |
|
-
- Annexure 2: Header & Footer specimen of IQMP
| Title: INSTRUMENT QUALIFICATION MASTER PLAN |
Page x of y |
|
| Document No.: |
CCR No. |
|
-
-
Annexure 3: Specimen Protocol Format
- Same as the above header
-
-
-
Annexure 4: Re-qualification of noncritical equipment
-
Purpose: To Re-Qualify the Equipment/ System after predefined time interval by review of the checklist
Name of Equipment / System :_____________________________
Equipment No. :_______________________________________
Location :____________________________________________
| Review Date: | Last Qualification Date: |
Review Checklist
Conclusion:____________________________
| Sr. No. | Checklist Details | Observation/ Comments | Reviewed By | Verified
By |
| 1. | Whether there is a significant change in any of the following that has influence on the quality of the product. | |||
| 1.1 | Replacement of any critical component.
(Provide Reference) |
|||
| 1.2 | Design change of any critical component. (Provide Reference) | |||
| 2. | Review of Utilization record. | |||
| 3. | Any other change that could have influence on quality of product (specify), based on equipment history card. | |||
Next Due Date : |
||||
| Reviewed By | Approved By | ||||
| Department Head | Maintenance Head | Production Head | QA | QA Head | Site Quality Head |
-
- Annexure 5: Flow Chart of Qualification Approach
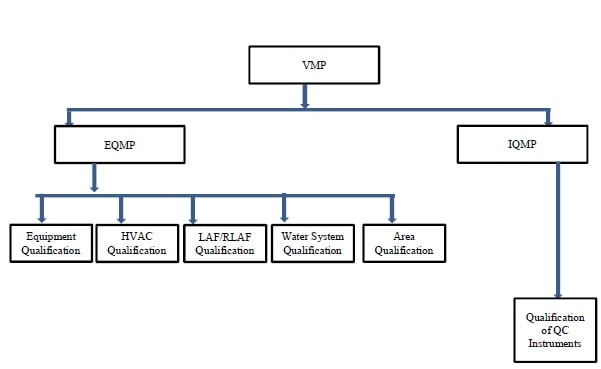
-
-
Annexure 6: Master List of Critical Equipment and their Qualification Status
- Prepare the list of critical Equipment by incorporating the following table content.
-
-
-
- Sr. No.
-
-
-
- Equipment ID
-
-
-
- Equipment Name
-
-
-
- Make
-
-
-
- Model
-
-
-
- Capacity
-
-
-
- Operating Range
-
-
-
- The last Calibration Done on
-
-
-
- Next Calibration Due on
-
-
-
- Location
-
-
-
Annexure 7: Master List of Non-Critical Equipment and their Qualification Status.
- As above – for the critical Equipment.
-
-
-
Annexure 8: Area Handover Certificate
-
On the basis of activity performed and the result finding during the Qualification of the Area_ _ _ _ _ _ _ _ _ _ _ _ _ _ _ from dated _ _ _ _ _ _ to _ _ _ _ _ _ , it is concluded that the area complies the area qualification.
Thus the Area has been hand over to _ _ _ _ _ _ _ _ _ _ department for routine batch processing into the same with effective from _ _ _ _ _ _ _ _ onwards.
Prepared By: Authorized By:
(Quality Assurance) (Site Quality Head)
-
-
Annexure 9: Equipment Handover Certificate
-
On the basis of activity performed and the result finding during the Qualification of the Equipment _ _ _ _ _ _ _ _ _ _ _ of Code No. _ _ _ _ __ from dated _ _ _ _ _ _ _ to _ _ _ _ _ _ , it is concluded that the equipment is performing as it was intended for.
Thus the Equipment has been hand over to _ _ _ _ _ _ _ _ _ _ department for routine batch processing into the same with effective from _ _ _ _ _ _ _ _ onwards.
Next Qualification due on: _ _ _ _ _ _ _ _
Prepared By: Authorized By:
(Quality Assurance) (Site Quality Head)
-
-
Annexure 10:Vendor’s Qualification Protocol Approval Certificate
-
Equipment Name:_______________________________
Model No: ____________________________________
Equipment Code: _______________________________
Vendor:______________________________________
Capacity: _____________________________________
This is to certify that the Vendor’s Qualification Protocol complies and meets the requirements as per Organization Standards.
Hence this Qualification Protocol reflects that the Qualification activity to be executed for the particular equipment shall be accepted as per the requirement.
| Reviewed By: | Approved By: | |||
| User | Engineering | QA | QA Head | Site Quality Head |



Pingback: Vendor Assessment / Evaluation Checklist - Guidelines - SOPs
Pingback: Environmental Monitoring (EM) - New Approach Guide - Pharma Beginners
Pingback: Sterilizing and Depyrogenating Tunnel - PQ Protocol - Pharma Beginners