Standard Operating Procedure (SOP) and Guideline to describe the process and requirements to file a Field Alert Report (FAR) to the U.S. Food and Drug Administration (FDA) for products distributed/marketed in the United States.
SOP for Field Alert Report Submission
1.0 OBJECTIVE
-
- This SOP/Guideline describes the process and requirements to file a Field Alert Report (FAR) to the U.S. Food and Drug Administration (FDA) for products distributed/marketed in the United States.
2.0 SCOPE
-
- This SOP/Guideline applies to the reporting requirements, as outlined in 21 CFR 314.81 (b)(1)(i)(ii) NDA Field Alert Report (FAR).
-
- This SOP/Guideline describes the process to submit the current Form FDA 3331 and any other subsequent communications to the FDA for drug products distributed to the U.S. market.
-
- Field Alert Report (FAR) shall be filed if a quality event impacts the identity, safety, purity, and quality of the product, as detailed in 21CFR 314.81 (b)(1)(i)(ii).
-
- Field Alert Report (FAR) are required to be submitted to the US FDA, for both confirmed or unconfirmed problems meeting the definition of the regulation as detailed below:
-
-
- Information concerning any incident that causes the drug product or its labeling to be mistaken for, or applied to, another article.
-
-
-
- Information concerning any bacteriological contamination, or any significant chemical, physical or other change or deterioration in the distributed drug product or any failure of one or more distributed batches of the drug product to meet the specification established for it in the application.
-
-
- This procedure applies to distributed NDA and ANDA of Products (including those manufactured at a third-party contract manufacturing site) as well as those products marketed in the United States.
-
- Compliance with this SOP / Guideline is mandatory, and this SOP / Guideline is applicable globally to all company’s products marketed to the US market.
-
-
EXCLUSIONS:
- Research & Development (R&D) activities are excluded from the scope of this SOP / Guideline.
-
-
- The requirements for reporting Biological Deviation Reports (BDPR) to the FDA are excluded from the scope of this SOP / Guideline.
3.0 RESPONSIBILITY – SUBMISSION OF FIELD ALERT REPORT :
-
-
Quality Assurance Head shall be responsible for:
-
-
- Notifying the –Quality-Americas of the Quality event within the 1st working day (Day-1) for evaluation/determination of Field Alert Report (FAR) applicability
-
- Notifying Head-Corporate Quality Compliance (CQC) in case the CAPA initiated for Field Alert Report (FAR) investigations may have any global impact.
-
- Initiating a formal investigation, by electronic means (in Track wise or an equivalent system) or where the manual system is followed, by the 3rd working day (Day-3) for Regulatory Head – US to submit the initial Field Alert Report (FAR) to the FDA.
-
- Implementing immediate actions such as blocking affected batches, making arrangement for the performance of spot checks,
-
- Implementing any additional controls required in the warehouse in cases where OOS test results or complaint investigations may impact the identity, safety, purity, strength, and quality of the product.
-
- Issuing Quality Alerts in a timely manner for any complaint, investigation, or OOS test results that may impact the identity, safety, purity, strength, and quality of the product, as detailed in 21 CFR 314.81 (b)(1)(i)(ii).
-
- Investigating, testing, and resolving Quality related events, and for the evaluation and assessment of the impact of these on other batches and/or products.
-
-
Regional Quality Head – Americas / Designee shall be responsible for :
-
-
- Receiving communication from the manufacturing sites, such as the investigation/deviation reports, stability results, product complaints, etc.
-
- Evaluation and determination of Field Alert Report (FAR) applicability under 21 C.F.R.§ 314.81(b)(1)(i) and (ii) for any applicable/warranted incident as per this standard.
-
- Communicating the Field Alert Report (FAR) submission decision taken to the Head Quality and Compliance, Regulatory Head, Manufacturing Head, and the Managing Director.
-
- Guiding the manufacturing site and Quality teams to meet Health Authority expectations.
-
-
Head of Quality and Compliance / Head Of Manufacturing / Regulatory Head / Managing Director (Or Respective Designees) shall be responsible For:
-
-
- Reviewing Field Alert Report (FAR) in conjunction with other relevant stakeholders, requesting clarification, as needed, and making recommendations to address the issue.
-
- Providing feedback to the Regional Head of Quality – Americas in the event of any disagreement with Regional Heads’ decision on Field Alert Report (FAR).
-
- Recommending Subject Matter Experts (SMEs) to the sites.
-
- Evaluating the multi-site impact of the CAPAs, if any, and monitoring and tracking the progress of the same.
-
- Ensuring that proper health authorities are notified within the required timeframe of a reportable incident related to a Quality Alert and that information is available to Quality Assurance, Drug/Device Safety Surveillance, and Regulatory Affairs.
-
-
Distribution / Warehouse Head or Designee shall be responsible for:
-
-
- Placement of affected batches in a quarantine hold status until the investigation is completed and a disposition has been applied to the quarantined batch.
-
-
Regulatory Affairs Head –Us / Designee shall be responsible for:
-
-
- Evaluation and determination of Field Alert Report (FAR) applicability under 21 C.F.R.§ 314.81(b)(1)(i) and (ii) for any applicable/warranted incident as per this standard.
-
- Receiving communication from the manufacturing sites, such as the investigation/deviation reports, stability results, product complaints, etc.
-
- Filing a Field Alert Report (FAR) with the appropriate FDA division and providing any subsequent updates received from the manufacturing sites to the FDA Agency for the product(s)/batch(s) that have been distributed to the U.S. market.
-
- Maintaining Field Alert Report (FAR) log for each FAR submitted to the Agency.
-
-
Drug Safety / Pharmacovigilance (PVG) / Medical Affairs Head or Designee shall be responsible For:
-
-
- Notification of any market complaint that may impact the
-
-
- Identity,
-
-
-
- Safety,
-
-
-
- Purity,
-
-
-
- Strength, and quality of the product
-
-
- within the 1st working day (Day-1) to the Quality Head-Americas and /or Regulatory Head – US for evaluation/determination of Field Alert Report (FAR) applicability.
-
- Performing Health Hazard Evaluation (HHE) assessment if the quality event is life-threatening (death has or could occur), permanent impairment of body function or permanent damage requires medical or surgical intervention, temporary or reversible (without medical intervention) or any adverse health consequences.
4.0 ABBREVIATIONS AND DEFINITIONS:
-
-
ANDA – Abbreviated New Drug Application:
-
-
- An application for a U.S. generic drug approval for an existing licensed medication or approved drug.
-
-
API – Active Pharmaceutical Ingredient:
-
-
- Any substance or mixture of substances intended to be used in the manufacture of a drug (medicinal) product and that,
-
- When used in the production of a drug, becomes an active ingredient of the drug product.
-
- Such substances are intended to furnish pharmacological activity or other direct effects in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or function of the body.
-
-
Assignable (Root) Cause:
-
-
- The origin or source of a quality event that has been identified to cause the defect.
-
- The most basic reason, which if eliminated, would prevent a recurrence.
-
-
Confirmed Event:
-
-
- Data or results generated outside of the pre-established expected range or acceptance criteria that are in agreement with the original result.
-
- A confirmed event shall be investigated, documented, reviewed, and approved by the site Quality Unit.
-
-
Controlled Document:
-
-
- A controlled document is a reference document that, through the course of its lifecycle may be reviewed, modified, and distributed several times.
-
-
Field Alert Form:
-
-
- A report submitted to the FDA outlined in the Code of Federal Regulation 314, Subpart 314.81, documented on Form FDA 3331.
-
-
HHE – Health Hazard Evaluation:
-
-
- An assessment to establish the potential risk associated with the use of a product associated with a potential quality event.
-
- Not limited to considering the population at risk, conditions that may exacerbate or attenuate the risk of occurrence, and the likelihood of the risk occurring in the future.
-
-
NDA – New Drug Application:
-
-
- The NDA application is the vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for sale and marketing in the U.S.
-
-
Un-confirmed Event:
-
-
- Original data or results that are generated outside of the pre-established expected specification range or acceptance criteria.
-
- A non-confirmed quality event shall be investigated, documented, reviewed, and approved by the Quality Unit.
-
-
OOS-Out-of-Specification:
-
-
- All results that fall outside the pre-established specifications or acceptance criteria established in the drug application, drug master files, and official compendia or by the manufacturer.
-
- An OOS shall be investigated, documented, reviewed, and approved by the site Quality Unit.
-
-
Significant Change:
-
-
- A change that could reasonably be expected to affect the safety or effectiveness of the product.
-
-
Quality Alert:
-
-
- General deficiency in systems, practices, or processes for GMP-QA areas that adversely affect,
-
- The safety or well-being of patients or public health and/or the quality, safety, and efficacy of medicinal products and/or that represent significant violations of applicable legislation and guidelines which may need to be informed/escalated to Senior Management of Company for information/support in resolution.
5.0 WORKFLOW – FIELD ALERT REPORT :
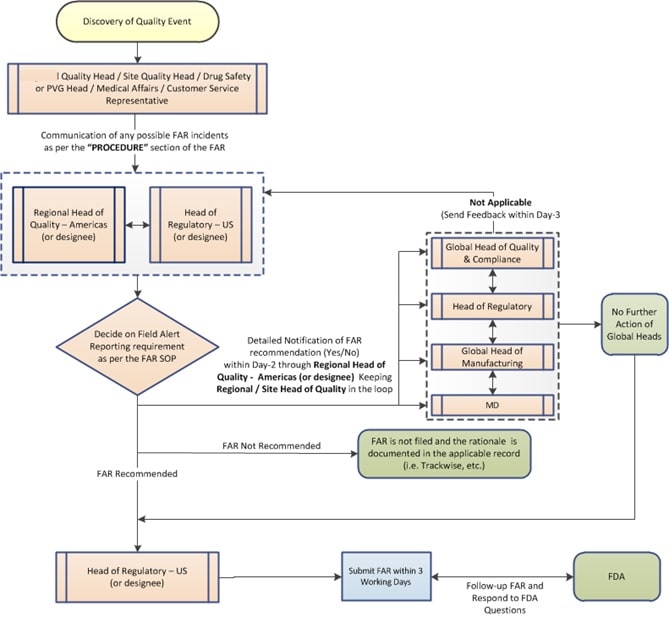
6.0 PROCESS – SUBMISSION OF FIELD ALERT REPORT:
-
- Field Alert Report (FAR) needs to be filed whenever the manufacturing site generates a quality event that complies with the FAR requirements 21 CFR 314.81 (b)(1)(i)(ii) .
-
-
The types of quality events requiring Field Alerts include, but are not limited to:
-
-
-
- Any incident that causes the drug product or its labeling to be mistaken for, or applied to, another article (Adulterated or misbranded). Examples include, but are not limited to:
-
-
-
-
- Labeling mix-up or errors
-
-
-
-
-
- Incorrect bottle label
-
-
-
-
-
- Incorrect insert
-
-
-
-
-
- Conflicting information on the label or insert
-
-
-
-
-
- An incorrect expiration date that allows a product to be distributed beyond its intended expiration date.
-
-
-
-
-
- Foreign product
-
-
-
-
-
Information concerning any bacterial contamination. Examples include, but are not limited to:
-
-
-
-
-
- Reported gross contamination
-
-
-
-
-
- Visible mold growth
-
-
-
-
-
Any significant chemical, physical, or other change or deterioration in the distributed drug product. Examples include, but are not limited to:
-
-
-
-
-
- Significant spots on tablets
-
-
-
-
-
- Significant discoloration or decomposition
-
-
-
-
-
- Crumbling of tablets
-
-
-
-
-
- Precipitate in a solution
-
-
-
-
-
- Hazardous substances, such as glass, metal, strong foreign odor.
-
-
-
-
-
Any failure of one or more distributed batches of the drug product to meet the specifications established for it in the application. Examples include, but are not limited to:
-
-
-
-
-
- An out-of-specification result generated during stability and/or a retain sample (reserve sample) analysis within the product expiration period.
-
-
-
-
-
- Whereby retention sample examination results for an unexpired distributed lot indicate an issue with container-closure integrity, label/labeling, contamination, deterioration, or otherwise not meeting specifications.
-
-
-
-
-
- Customer complaints where preliminary information and investigation indicate the likelihood of an issue with the batch unrelated to mishandling by the customer. (Refer to SOP for Market Complaints.)
-
-
-
-
- Deviations / Investigations that identify a root cause and scope of potential impact implicating a distributed batch.
-
-
-
Process of Reporting the Investigation – Field Alert Report:
-
-
- The Quality Head shall communicate any concerns that are identified for immediate consideration of filing a Field Alert Report (FAR) based on the examples provided above to the Head of Quality – Americas and/or Regulatory Head – US within the 1st working day (Day-1) for their evaluation/determination of Field Alert Report (FAR) applicability. (Refer Attachment-II)
-
- The Quality Head shall initiate and conduct their investigation following the procedures described in the SOP for Investigations.
-
- A description of the incident and any preliminary investigation, supporting documentation, root cause, corrective and preventative actions, etc.
-
- In addition, the Quality Head will also submit the investigation report and CAPAs to Corporate Compliance.
-
- Corporate Compliance shall review and evaluate the multi-site impact of CAPAs and take necessary actions if applicable.
-
-
Actions prior to Submission of Field Alert Report :
-
-
- Additionally, Corporate Compliance shall provide the feedback to the Regulatory Head – the US, if any, before Field Alert Report (FAR) submissions. (Refer to SOP for Handling of Corrective and Preventive Actions -CAPA).
-
- The Head of Quality-Americas and the Regulatory Head – US will discuss and take a risk-based decision to conclude the recommendation for field alert reporting requirement, i.e., whether a Field Alert needs to be filed under 21 C.F.R.§ 314.81(b)(1)(i) and (ii).
-
- Subsequently, the Regional Head of Quality –Americas shall communicate the Field Alert Report (FAR) submission decision taken (irrespective of whether FAR is recommended or not) to the
-
-
- Head Quality and Compliance,
-
-
-
- Manufacturing Head,
-
-
-
- Head Regulatory Affairs and
-
-
-
- The Managing Director as early as possible within the 2nd working day (Day-2) keeping the Regional / Site Quality Head in the loop.
-
-
-
Actions after confirmation to Filing of Field Alert Report (FAR):
-
-
- Once notified on the Field Alert Report (FAR) submission decision,
-
- All Heads shall be responsible for sending the feedback to the Head of Quality –Americas within the 3rd working day (Day-3), only in the event of any disagreement with the Regional Quality Heads’ Field Alert Report (FAR) decision.
-
- The FARs submitted to the FDA are classified as Initial, Follow-up, or Final reports.
-
- Regulatory Head – US shall notify the FDA by telephone, by mail or other rapid communication within three (3) working days of the company becoming aware of the reported problem or event that requires a Field Alert Report (FAR), as detailed in 21 CFR 314.81 (b)(1)(i)(ii) for the Initial FAR.
-
- The Initial Field Alert Report (FAR) shall include an assessment of the cause of the event based on the information available at the time.
-
- If the initial rapid communication did not include transmittal of the information via the current version of Form FDA 3331, the Regulatory Head – US (or designee) will follow up the initial communication in writing via submission of FDA Form 3331 (refer Attachment – II) to the FDA District Office.
-
- Commitments made must be documented and tracked to completion and made available to the FDA, if requested.
-
- A follow-up Field Alert Report (FAR) will be submitted by the Regulatory Head – US to the FDA every thirty (30) calendar days until the investigation is completed and the Final FAR is filed.
-
-
The follow-up FARs shall identify the progress made in the investigation.
-
-
- The Final Field Alert Report (FAR) will be submitted by Regulatory Head – US to the FDA, not more than five (5) working days upon receipt of the concluded (finalized) investigation report from the manufacturing sites.
-
- If there is an unforeseen delay that is encountered to submit the final report within five (5) working days, this incident shall also be documented in the Field Alert Final Report.
-
- Drug Safety/Pharmacovigilance (PVG) /Medical Affairs may perform a Health Hazard Evaluation (HHE) to assess the impact of the quality event regarding the identity, safety, purity, strength, and quality of the product for patient safety.
-
- Include the assessment summary in the Field Alert Report (FAR) correspondence to the FDA, as submitted by Regulatory Affairs.
-
- Track the complete cycle of events in the Field Alert Report (FAR) log (refer to attachment-III), maintained by the US Regulatory Affairs. All FDA Filed Alert communications and log the submissions in that log.
7.0 REQUIREMENTS – SUBMISSION OF FIELD ALERT REPORT:
-
-
Completing a Field Report Form 3331:
-
-
- FARs involving foreign facilities can be reported to the jurisdictional FDA Office, where the firm’s attorney, agent, or other authorized official resides or maintains a place of business in the U.S.
-
- The Field Alert Report (FAR) form contains an expiration date in the top left-hand corner of the form. Ensure the current FDA Form 3331 is utilized.
-
- The FDA website for FDA forms is found at http://www.fda.gov/aboutfda/reportsmanualsforms/forms/listformsalphabetically/default.htm
Note: As an option, the U.S. FDA has launched a pilot program to electronically submit FARS using Form FDA 3331A (Automated) to improve the speed and efficiency of reporting quality issues relating to the manufacture of FDA-approved drug products.
Instructions are provided online for use of Form 3331A. For details see http://www.fda.gov and enter 3331A.
-
- Follow the Field Alert Report (FAR) instructions supplied in Attachment II.
-
- Complete the electronic Field Alert Report (FAR) Form 3331 online, save a copy to your computer, and print two (2) copies of the form.
-
- Submit a separate narrative report with Form 3331 describing the event, summarizing the investigation, and the Health Hazard Evaluation, including any corrective action taken for the affected product.
-
-
Field Alert Report (FAR) LOG:
-
-
- The log may be a bound book, paginated, and properly labeled for its intended purpose, or an electronic version of the same, provided it is managed as a controlled document. (refer to attachment-III)
-
- The log shall contain the following headers at the minimum (but not limited to) :
-
-
- Product Name/Potency
-
-
-
- Batch Number
-
-
-
- Batch Manufacturing and Expiry Date
-
-
-
- Pack style
-
-
-
- ANDA/NDC No.
-
-
-
- Reference Document Number
-
-
-
- Complaint Number / OOS Number
-
-
-
- Application Number
-
-
-
- Manufacturing Site
-
-
-
- Field Alert Report (FAR) Description
-
-
-
- Receiving Date
-
-
-
- Date notified to FDA (Initial)
-
-
-
- Date of notification of Follow-up report
-
-
-
- Field Alert Report (FAR) Report Type
-
-
-
- Status
-
-
- Retain all FDA Field Alert communications and submissions as per the record retention procedures.
8.0 ATTACHMENTS – FIELD ALERT REPORT (FAR):
Attachment I – Example Template of OOS/Deviation/Incident Notification Form
| OOS/Deviation No: | Date: | Location: | |
| From:
Regional / Location (Site) Head of Quality |
To: Regional Head of Quality – Americas |
||
OOS/Deviation/Incident of the following Batch (es) recommended:……………………………………..
| S. No. | Product | Batch Number | Manufacturing Date | Expiry Date |
OOS/Deviation Details:………………………………………………………………………………
Attachment II – NDA FDA Form 3331
| DEPARTMENT OF HEALTH AND HUMAN SERVICES | TO: (NAME AND ADDRESS OF DISTRICT) | |
| FOOD AND DRUG ADMINISTRATION | ||
| NDA-FIELD ALERT REPORT | ||
| TYPE OF REPORT | ||
| In accordance with section 314.81(b)(1)(i) and (ii) of the New Drug Application Regulations (21 CFR 314) promulgated under the Federal Food, Drug and Cosmetic Act, as amended, the following is herewith submitted: | ||
| 1. NDA/ANDA | 2. NDC No. | |
| 3. GENERIC NAME OF DRUG PRODUCT | 4. TRADE / BRAND NAME (if any) | |
| 5. FIRM NAME AND ADDRESS WHERE PROBLEM OCCURRED | 6. FEI/CFN | |
| 7. DOSAGE FORM, STRENGTH AND PACKAGE SIZE(S) | ||
| 8. LOT NUMBER(S) | ||
| 9. EXPIRATION DATE(S) OF DRUG PRODUCTS | ||
| 10. DATE WHEN NOTIFIED ABOUT PROBLEM(S) OR WHEN PROBLEM(S) FIRST BECAME KNOWN TO APPLICATION HOLDER | ||
| 11. HOW WAS PROBLEM DISCOVERED | ||
| 12. STATE PROBLEM(S) | ||
| 13. ROOT CAUSE(S) OF PROBLEM(S) | ||
| 14. CORRECTIVE ACTION(S) TAKEN (if any) TO PREVENT RECURRENCE OF PROBLEM(S) | ||
| 15. REMARKS | ||
| NOTE: SEPARATE NARRATIVE REPORTS MAY BE ATTACHED IF DESIRED | ||
| REPORTING ESTABLISHMENT | ||
| NAME AND MAILING ADDRESS (include ZIP Code) | ||
| NAME AND TITLE OF AUTHORIZED REPRESENTATIVE | TELEPHONE (Include Area Code) | |
| SIGNATURE OF AUTHORIZED REPRESENTATIVE | DATE SUBMITTED | |
Attachment III – Field Alert Report (FAR) Log
| ANDA/ NDA | Name of Drug Product | NDC | Strength | Pack Size(s) | Lot # | Facility | Date Notified | Date: Initial Report |
| Follow-up Report 1 | Report 2 | Report 3 | Date: Follow-up Report 4 | Date: Final Report | Description | Status |
9.0 REFERENCES
-
- 21 CFR Part 314: Application for FDA Approval to Market a New Drug, Subpart B: Applications 314.81: Other Post-Marketing Reports, NDA FAR (b)(1)(i)(ii)
-
- New Drug Application (NDA) FARs Filing an NDA Field Alert, FDA Guidance Document
-
- Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production, FDA Guidance Document, issued October 2006
-
- Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients, ICH Guideline, issued August 2001
-
- Current FDA Form 3331 Website “FDA Forms”
-
- Guideline for Investigations Tools Used in Pharma
-
- SOP for Market Complaint.
**********************************************END**********************************************


