Guidance (SOP) about the procedure and documentation of reprocess and rework during Active Pharmaceutical Ingredient (API), excipients, and Drug Product in Pharmaceuticals.
Guideline for Reprocess / Rework of API & Drug Product
1.0 Purpose :
-
- The purpose of this guideline is to describe the specific requirements for reprocessing and reworking of active pharmaceutical ingredients (API) and excipients.
-
- The guideline also describes the requirement for re-labeling/re-packaging for Drug Products to ensure they are managed and performed in a controlled and compliant manner.
2.0 Scope :
-
- This guideline applies to all commercial and regulatory submission batches.
-
- This guideline applies to cGxP Reprocess/Rework activities, documentation, and records that execute the requirements as outlined in this guideline.
-
- Re-labeling and Re-packaging operations are within the scope of this document.
-
-
Exclusions :
-
-
- Research & Development functions are excluded from the scope of this guideline.
-
- Specific requirements for Contractors and Suppliers will be detailed in Service Agreements, Quality Agreements, and/or Associated Procedures.
-
- Validation of reprocessing and rework for Development materials is not required
3.0 Responsibility – Reprocess and Rework
-
-
Quality Assurance Head shall be responsible for –
-
-
- Ensuring reprocess and rework activities are completed in accordance with this guideline/SOP.
-
- Monitoring compliance in accordance with this guideline/SOP.
-
- Approving re-labeling and/or repackaging documentation plan, Batch Record, and/protocol, as applicable, prior to execution.
-
- Assessing and approving/rejecting the change control for reprocessing/reworking.
-
- Reviewing and approving all reprocess and rework activities through the protocol, batch record, investigations, and/or Change Control prior to implementation.
-
- Reviewing and approving corrective and preventative actions (CAPA) developed during the reprocess/rework deviation/incident investigation. Assess and report the effectiveness of implemented CAPA.
-
- Investigating reprocess/rework deviation/incident events.
-
- Reporting all site rework/reprocessing activities.
-
- Assessing the need to implement reprocessing /reworking procedure as part of the established process.
-
- Notifying Regulatory Affairs Head/designee about proposed reprocessing or reworking steps for potential impact on regulatory filings.
-
-
Manufacturing Head shall be responsible for :
-
-
- Ensuring adequate resources, facilities, and equipment are available to perform relabeling/repacking operations in compliance with cGxP.
-
- Ensuring relabeling and repackaging materials comply with requirements established in the product registration, monograph, or equivalent.
-
- Labeling and packaging materials that are removed are reconciled and destroyed in a controlled manner such as to render them useless.
-
- Identifying, communicating, and investigating reprocess/rework deviation/incident events in collaboration with the QA.
-
- Assisting the Quality Unit to investigate the deviation/incident that led to the need for reprocessing or reworking.
-
- Initiating a change control, if necessary, to manage the reprocess and rework activities for in-process materials, problem batches of raw materials/components.
-
- Completing the rework or reprocess operations in accordance with this guideline.
-
- Implementing CAPA for the investigations to address the identified issue and avoid its recurrence.
-
-
Regulatory Affairs Head shall be responsible for:
-
-
- Reviewing and documenting the potential impact of reprocessing/rework activities on the regulatory requirements for the markets served.
-
- Advising the QA Head/designee about mandatory regulatory requirements and whether or not the reprocessing or reworking should be reported and submitted to the appropriate regulatory authorities (Drug Master File, Annual Product Review / Product Quality Review).
-
- Providing appropriate review and guidance to the QA Head/designee regarding regulatory submission filing requirements related to requests for reprocessing and reworking (Drug Master File, Annual Product Review/Product Quality Review, etc.). Complete revised regulatory filings in the markets served in a timely manner, as required.
4.0 Definition of Terms ( Reprocess and Rework)
-
-
Definition of API (What is API) :
-
-
- API Active Pharmaceutical Ingredient is an ingredient intended to furnish pharmacologic activity or other direct effects in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the body; it does not include intermediates used in the synthesis of
such an ingredient.
- API Active Pharmaceutical Ingredient is an ingredient intended to furnish pharmacologic activity or other direct effects in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the body; it does not include intermediates used in the synthesis of
-
-
Definition of Batch (or Lot)
-
-
- A specific quantity of material produced in a process or series of processes so that it is expected to be
homogeneous within specified limits.
- A specific quantity of material produced in a process or series of processes so that it is expected to be
-
-
Definition of Batch Record (What is Batch Record)
-
-
- Batch Record All documents associated with the manufacture of a batch of API, bulk product, or finished product. The BR/BPR is a controlled document that provides a historical record of each batch manufactured and all circumstances pertinent to the quality of the batch. It includes the formulation, theoretical yield, manufacturing procedures, equipment and processes used, packaging components, assay and in-process testing requirements and results, date and time steps that are completed, sampling, and labeling of batches or production lots. (Also refer to Master Batch Record.)
-
-
What is cGxP
-
-
- cGxP is a general term that stands for current Good “x” Practice (x = Clinical, Engineering, Distribution, Laboratory, Manufacturing, Documentation, Pharmaceutical, etc.). The titles of these good “x” practice guidelines usually begin with ‘Good’ and end in ‘Practice’. cGxP represents the abbreviations of these titles, where x (a common symbol for a variable) represents the specific descriptor.
-
What is Change Control :
- Change control is another well-known cGMP concept that focuses on managing change to prevent unintended consequences. The CGMP regulations provide for change control primarily through the assigned responsibilities of the quality control unit. Certain major manufacturing changes (e.g., changes that alter specifications, a critical product attribute, or bioavailability) require regulatory filings and prior regulatory approval (21 CFR 314.70, 514.8, and 601.12).
-
What is Deviation :
- Any departure (planned or unplanned) from approved procedures or records, including, but not limited to Standard Operating Procedure, Master Production Record, Batch Production Record, Standard Testing Procedure, or the failure of a batch or any of its components to meet any of its specifications shall be documented and explained. Potential product quality impacting events shall be investigated and the investigation and its conclusions shall be documented (SOP for Deviation Management)
| Event | Any unforeseen happening or unexpected occurrence. |
| ID | Identify. |
| Intermediate | A material produced during steps in the synthesis of an API that must undergo further molecular change or processing before it becomes an API |
| LOD | Loss on Drying |
| Material | Any finished product, semi-finished product, API, raw material, Intermediate, Key Starting Material or sample |
Packaging Material |
Any printed and unprinted material employed in the packaging and delivery process of a supply chain material that is intended to provide information or protection during its storage and transportation. Primary packaging materials have direct contact with the drug substance. Secondary packaging materials do not have direct contact with the drug substance. Tertiary packaging materials are used to transport the primary and secondary packaging (i.e. corrugated shipping containers). Temperature-Controlled Shippers are used, where required, based on labeled storage conditions of the product to control temperature during the shipping of drug substances and include only passive shipping. |
| Primary Packaging | First level product packaging that comes in direct contact with the product and works as a barrier between the product and environment. |
| Quarantine | A status indicating material or finished product is awaiting final disposition by the Quality Assurance, for example, material for which inspection and testing are not yet completed or material that is under investigation. Quarantined materials are blocked by the Quality Assurance and cannot be used for further processing. |
| Rejected Material | Material that has not passed approved specification requirements and will be returned to the supplier, reprocessed after being reviewed and approved by the Quality Assurance, or destroyed in a controlled manner. |
| Rendered Useless | GMP materials destined for destruction are rendered useless (not fit for use by ordinary means). |
| Reprocessing | Introducing an intermediate or API, including one that does not conform to standards or specifications, back into the process and repeating a crystallization step or other appropriate chemical or physical manipulation steps (e.g., distillation, filtration, chromatography, milling) that are part of the established manufacturing process. |
Rework |
Subjecting an intermediate or an API that does not conform to standards or specifications to one or more processing steps that are different from the established manufacturing process to obtain acceptable quality intermediate or API (e.g., recrystallizing with a different solvent). |
| SME | Subjecting an intermediate or an API that does not conform to standards or specifications to one or more processing steps that are different from the established manufacturing process to obtain acceptable quality intermediate or API (e.g., recrystallizing with a different solvent). |
| Validation (Facility, Equipment, Utility) | The activity of establishing documented evidence, which provides a high degree of assurance that a specific method, process, or system will consistently produce a result meeting. its predetermined specifications and quality attributes. |
5.0 Flow Chart ( Reprocess and Rework)
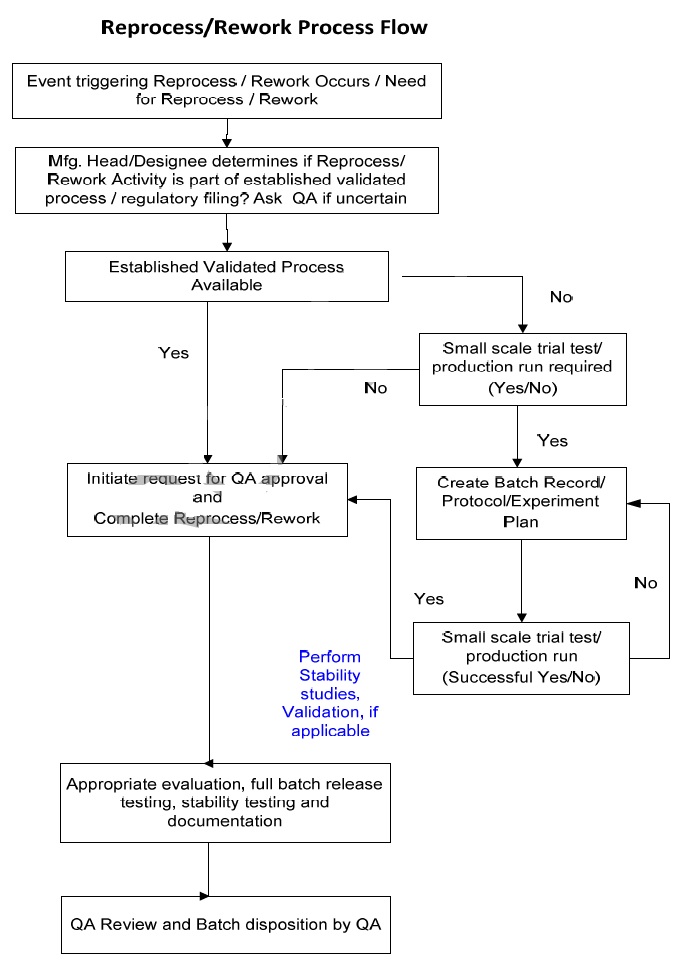
5.0 Procedure – Reprocess and Rework :
- In-process non-confirming API, Intermediate, Raw Materials, and Components:
- The MFG Head/designee shall determine whether the corrective action needed to bring the lot back into conformance shall be completed following the steps listed below:
-
-
- By continuing or repeating existing process steps.
-
-
-
- That the process step(s) to be continued or repeated are included in the process/production validation and process filing and are considered to be part of the normal process.
-
-
-
- Should the MFG Head/designee be uncertain about the response to either step above, then they shall confer with the QA Head/designee to make a determination.
-
-
- If the answer to both these questions is with a “yes” and validated
the process is available, MFG Head/designee shall inform Site Head-QA/designee each time reprocessing / reworking is required such that he/she:
- If the answer to both these questions is with a “yes” and validated
-
-
- Shall review the decision made by the Manufacturing.
-
-
-
- Shall monitor and report reprocessing/reworking activity.
-
-
- The MFG Head/designee shall inform the QA Head/designee each time reprocessing / reworking is required such that:
-
-
- QA Head/designee shall review the decision made by the Manufacturing Unit.
-
-
-
- QA Head/designee shall monitor and report reprocessing/reworking activity.
-
-
-
The following activities together shall be used to develop and implement the Reprocess or Rework Plan:
-
-
-
- Document supporting rationale to justify implementing reprocessing or reworking.
-
-
-
- Detailed reprocessing or rework procedures, steps, and validation, if appropriate.
-
-
-
- Document any new in-process and finished product testing requirement, as appropriate.
-
-
-
- Compare impurity profiles of material made with the current process versus the revised reprocess or rework process.
-
-
-
- Document critical processing parameters, as appropriate, needed for the process to remain in control and meet specifications.
-
-
-
- Determine requirements to revise regulatory filings for the affected markets.
-
-
-
- If the rework and reprocess activities are being executed with regularity and recurring frequency, then a change control should be initiated to incorporate these rework or reprocessing steps into the routine manufacturing process. QA Head/designee shall request the Manufacturing Unit to initiate a Change Control in accordance with the current version of SOP – Change Control Management.
-
-
-
-
The Regulatory Unit shall be included in the Change Control process to address any changes to the filed process.
-
-
-
- If the MFG Head/designee cannot answer both questions with a “yes,” consideration shall be given to performing a small-scale trial test following the Plan developed for the reprocess/rework process. The
decision about whether or not to conduct a test trial shall be justified in writing with supporting rationale provided by MFG Head/designee and/or R & D and be reviewed/approved by QA Head/designee
- If the MFG Head/designee cannot answer both questions with a “yes,” consideration shall be given to performing a small-scale trial test following the Plan developed for the reprocess/rework process. The
-
-
- The trial test should be documented by Protocol or Batch Record or Experiment Plan to define the reprocessing or rework procedure, how it will be carried out, and the expected results. Trial results will be documented, analyzed, and evaluated.
-
-
- Alternatively, the MFG Head/designee and R & D may request to proceed directly to small-scale production, operated under a Batch Record or Protocol to define the reprocessing or rework procedure, how it will be conducted, and the expected results.
-
-
The MFG Head/designee and PD shall prepare a justification that is reviewed and approved by the QA Head/designee, with supporting rationale for why the use of a small-scale trial test is not required.
-
-
- Batches that have been reprocessed or reworked shall be subjected to appropriate evaluation, full batch release testing, stability testing and documentation to demonstrate that the reprocessed or reworked
product is of equivalent quality to the product made following the original process.
- Batches that have been reprocessed or reworked shall be subjected to appropriate evaluation, full batch release testing, stability testing and documentation to demonstrate that the reprocessed or reworked
-
- A risk assessment shall be completed to determine the level of validation required but as a minimum requirement, concurrent validation shall be required for reprocessing / reworking.
-
- A protocol / Batch Record shall define the reprocessing or rework procedure, how it will be carried out, and the expected results.
-
- Regulatory Affairs Unit shall update and submit regulatory submission filings to the appropriate regulatory authorities covering the revised reprocessing or rework procedures. Reprocessed / Reworked lots can only be supplied once regulatory approvals are in place.
-
- QA shall make the final disposition decision for the reprocessed or reworked batch. The release of the first-time reprocess batch shall be done based on the evaluation of 3 months accelerated stability data with the same Shelf life/Retest period of the product. Where required, the batch disposition decision may be taken based on an additional study for 10 days at 60 Deg.C.
-
-
PROCEDURE FOR API ASSIGNED WITH EXPIRY OR RETEST DATES:
-
-
- All APIs that are assigned an expiry date shall be reprocessed after the expiry date. Where the API manufacturer has all related historical GMP documentation and additional stability data on the reworked or reprocessed API.
-
- The following shall be the objective of API reprocessing:
-
-
- For approved batches of unsold stock nearing retest/expiry date.
-
-
-
- For unsold stock beyond assigned retest/expiry date.
-
-
-
- Reprocessing of API before specific batch reaches assigned retest/expiry date (based on API label and supporting stability data).
-
-
-
-
- For example, API manufactured Jan 2021 with a retest/expiry date of December 2023.
-
-
-
-
-
- This batch can be reprocessed up until December 2021 and assigned the original full expiration period provided:
-
-
-
-
-
-
- Reprocessing is part of the validated process and is permitted in the Drug Master File, filed with the appropriate regulatory authorities.
-
-
-
-
-
-
-
- API is fully reprocessed (for example, recrystallized).
-
-
-
-
-
-
-
- This new retest/expiry date is supported by stability data based on the reprocessed material.
-
-
-
-
-
-
-
- Client/customer agrees with and is aware of this action, which should be documented in the Quality Agreement, as applicable.
-
-
-
-
-
Reprocessing of API after specific batch reaches assigned retest/expiry date (based on API label and supporting stability data).
-
-
-
- For example, API manufactured Jan 2021 with a retest/expiry date of Dec 2023.
-
-
-
- Reprocessing API that is past assigned retest/expiry date may be performed provided:
-
-
-
- The API is fully reprocessed (for example recrystallized).
-
-
-
- Reprocessing is part of the validated process and is permitted in the Drug Master File, filed with the
appropriate regulatory authorities.
- Reprocessing is part of the validated process and is permitted in the Drug Master File, filed with the
-
-
-
- The new retest/expiry date is supported by stability data based on the reprocessed material.
-
-
-
- Client/customer agrees with and is aware of this action, which should be documented in the Quality
the agreement, as applicable.
- Client/customer agrees with and is aware of this action, which should be documented in the Quality
-
-
- Initial 3 batches, which are reprocessed when approaching Retest/Expiry or after reaches assigned retest/expiry date shall be put on Accelerated and Real-time Stability Studies to re-define the
Retest/Shelf life period of Reprocessed batches for said reason.
- Initial 3 batches, which are reprocessed when approaching Retest/Expiry or after reaches assigned retest/expiry date shall be put on Accelerated and Real-time Stability Studies to re-define the
-
REQUIREMENTS:
-
- Reprocessing and rework shall not be performed without the review and approval of the Quality Head/designee.
-
- Out-of-specification batches having similar failure shall not be blended to reprocess together.
-
- Product returned from customers or product that has failed microbial testing shall not be reprocessed or reworked without QA evaluation.
-
-
Reprocess, Rework Material shall be:
-
-
- The repetition of final step or repletion of step by going back to process i.e. N-1 and then Final step (e.g. Salt back to base etc.)
-
- Processed under the same cGxP controls and conditions used for the original product.
-
- Monitored and trended as part of Annual Product Review.
-
- Segregated, clearly identified with appropriate status, and strictly controlled to prevent mix-ups.
-
- Have procedures supporting documentation, validation, testing, stability, and Regulatory Agency notification/approval, as required.
-
- Meet all specifications prior to disposition by the QA Head/designee.
-
- Have a batch record and/or protocol developed (if applicable) to document all activities. These documents shall be reviewed and approved by QA prior to the execution of activities.
-
-
Materials must be documented and traceable to the original batch/lot.
-
-
- Where applicable, batch/lots shall be included and tracked through the product stability program.
-
- If an API batch fails to meet the required specifications at initial testing after completing one (1), one more reprocessing is allowed with the same re-processing procedure.
-
-
- If reprocessing is routinely required, then it should become part of the filling process and regulatory
filing documents shall be updated in order to reflect the change in the established process.
- If reprocessing is routinely required, then it should become part of the filling process and regulatory
-
-
- Quality Unit shall monitor for out-of-trend (OOT) results recurring at the same processing step(s) which should be identified for consideration of reworking or reprocessing to improve the state of control.
-
- If reprocessing/reworking is used for a majority of batches, such reprocessing should be included as part of the standard manufacturing process through applicable change management procedures.
-
- The regulatory filing documents shall be updated in order to reflect the change in the established process.
-
-
STABILITY REQUIREMENTS:
-
-
- When more than one reprocessed or reworked API batch is affected, three initial batches at a minimum shall be put on Accelerated and Real-Time stability as and when the opportunity is available.
-
- A reprocessed or reworked batch originating from a known defect and where historical stability data is available shall be placed on routine long-term stability.
-
- The same reprocessing/rework procedure as in the past must have been applied for such a batch. The disposition of the affected lots/batches shall follow the routine batch release procedures.
-
- When reprocessing is required, due to minor defects of a batch, such as “extraneous matter” where only a repetition of the routine step, e.g. filtration and crystallization is applied, the need to place the batch on long term stability may be waived, however, the waiver must be documented, reviewed and approved by the QA Head/designee.
-
-
The QA Unit shall disposition the affected lots/batches following routine batch release procedures.
-
-
- In case the batch is reprocessed by repetition of the routine step, e.g. filtration and crystallization, the QA Unit may disposition the affected lots/batches following routine batch release procedures, based on the risk assessment document, reviewed and approved by QA.
-
- The evaluation shall also include the quality equivalence between reprocessed batch and normal batch.
-
- The QA Unit shall disposition the affected lots/batches following routine batch release procedures.
-
- When the rework procedure is different from the procedure applied previously for the same defect/defects an accelerated and long-term stability study shall be initiated.
-
- The accelerated stability study shall cover a three-month time period at a minimum and equivalence must be proven with a routine batch under the same stability conditions.
-
- The affected batch/batches shall be kept “quarantine” status until the accelerated stability study is completed and equivalence is demonstrated and approved by QA.
6.0 REFERENCES
-
- U.S. FDA 21 CFR Part 211
-
- International Society for Pharmaceutical Engineering (ISPE), Risk-Based Manufacture of Pharmaceutical Products, Volume 7, First Edition, September 2010
-
- European Commission EudraLex Volume 4 – Good Manufacturing Practice (GMP) Guidelines Chapters 1,4,5,6.
-
- Health Canada’s Good Manufacturing Practices Guidelines – 2009 Edition (GUI-0001)
-
- ICH (International Conference on Harmonization): Quality Guidelines
-
- Guidance for Pharmaceutical Manufacturers and Distributors (Orange Guide) 2015
-
- Pharmaceutical Inspection Convention and Pharmaceutical Inspection Cooperation Scheme (PIC/S): Guide to Good Manufacturing Practice for Medicinal Products
-
- United States Pharmacopoeia General Chapter 1078, Good Manufacturing Practices
-
- Change Control Management (SOP)
-
- Handling of Corrective and Preventive Actions (CAPA) (SOP)
-
- Deviations/Incidents (SOP)
-
- Investigations (SOP)
-
- Quality Risk Management (SOP)
************************************************END************************************************



Pingback: Quality Metrics - New FDA Guideline - Pharma Beginners