Protocol for Qualification and Re-Qualification or Validation of Heating Ventilation and Air Conditioning (HVAC) system for Clean Room.
HVAC System Qualification Protocol (Validation)
Table of Content – HVAC System Qualification Protocol
| S.No. | Table of Contents | Page |
| 1.0 | Protocol Approval | |
| 2.0 | History Sheet | |
| 3.0 | Objective | |
| 4.0 | Scope | |
| 5.0 | Responsibilities | |
| 6.0 | Equipment Description | |
| 6.1 | System Specification | |
| 7.0 | Requalification tests | |
| 7.1 | Air velocity/airflow measurement and calculation of air changes: | |
| 7.2 | Integrity Test Of HEPA Filter | |
| 7.3 | Differential Pressure Measurement | |
| 7.4 | Temperature And Humidity Control Test | |
| 7.5 | Air Flow Pattern Test | |
| 7.6 | Non- Viable Particle Count | |
| 7.7 | Recovery Study | |
| 7.8 | Passive Air Sampling | |
| 7.9 | Active Air Sampling | |
| 7.10 | Re-qualification Criteria | |
| 8.0 | Deficiency and corrective action report | |
| 9.0 | Requalification summary report | |
| 10.0 | Approval of requalification report | |
| 11.0 | List of forms/Annexure | |
| 12.0 | References | |
| 13.0 | Abbreviations |
1.0 Approval Sheet of Protocol:
| Prepared by: | ||||
|
Department |
Name | Designation | Signature |
Date |
| Quality Assurance | ||||
Related: SOP for Qualification of HVAC System
| Reviewed by: | ||||
|
Department |
Name | Designation | Signature |
Date |
| Engineering | ||||
| Concern | ||||
| Quality Control /Microbiology | ||||
| Quality Assurance | ||||
| Approved by: | ||||
|
Department |
Name | Designation | Signature |
Date |
| Head Quality Assurance |
|
|||
2.0 History Sheet :
| Rev. No. | Effective date | Nature of change/ reason for revision | Approved by QA |
3.0 Objective –HVAC System Qualification Protocol :
-
- The objective of this protocol is to provide an outline for the qualification of the HVAC system and to establish documentary evidence to demonstrate that the Air Handling Units (AHU’s) are qualified to perform well within the predetermined acceptance criteria of performance as per guideline outlined in this protocol.
4.0 Scope –HVAC System Qualification Protocol :
-
- The scope of this protocol is applicable for the requalification of Air handling unit (AHU) system,
5.0 Responsibilities –HVAC System Qualification Protocol :
|
Department |
Responsibilities |
|
| Quality Assurance | : |
|
| User department | : |
|
| Quality Control / Microbiology | : |
|
| Engineering | : |
|
| External agency | : |
|
Execution Team –HVAC System Qualification Protocol :
Execution team shall comprise of the representative from the following functions:
| Department | Name | Designation | Signature | Date |
| External agency | ||||
| Engineering | ||||
| Concern | ||||
| Microbiology | ||||
| Quality Assurance |
Pre Requisite:

-
- Daily cleaning of the area.
-
- Availability of area
-
- Required materials and instruments are available.
-
- Calibration and Preventive Maintenance of equipment.
-
- The gowning procedure of plant personnel and external agency shall be done as per the respective SOP for Entry and exit to the Aseptic area.
-
- Personnel qualification of the external party shall be done as per the respective SOP “Qualification of personnel for working in the aseptic area”.
-
- Personnel hygiene of personnel.
-
- Fogging cleaning shall be done prior to performing the requalification activity.
-
- External party agreement, respective valid SOP, and traceability certificates of instruments. Please elaborate. These are the bullet points.
-
- Training records of the external parties.
-
- Calibration of instruments or equipment used for testing like Anemometer, Aerosol photometer, Non-viable particle counter, etc.
6.0 Equipment Description / Specification:
-
-
System Specification:
-
-
- Details of AHU’s are as follow:-
|
Sr. No. |
AHU NO. | Area
(In m2) |
Room ID | Rooms Catered | Grade / ISO Grade | No. of HEPA | No. of Return Risers | Capacity
CFM |
ACPH
(NLT) |
Temp. (NMT)
(In oC) |
RH (NMT)
(In %) |
DP(In Pa) |
|
Design Parameters |
||||||||||||
7.0 Requalification Tests:-
Detailed below-mentioned parameters shall be performed in the requalification of AHU’s.
| Sr. No. | TESTS | RE-QUALIFICATION FREQUENCY |
| 01 | Air Velocity Measurement and Calculation of Air Changes |
|
| 02 | Integrity Testing y HEPA Filter |
|
| 03 | Pressure Differential Test |
|
| 04 | Temperature and Relative Humidity Test |
|
| 05 | Air Flow Pattern Test |
|
| 06 | Cleanliness Class Verification
Non Viable Particulate count. At-rest condition and in operation for ISO class 5, 6, 7, and 8 |
|
|
07 |
Microbial monitoring |
|
| 08 | Recovery Test |
|
-
Air velocity, the Air volume, and Air Changes Per Hour (ACPH) measurement:
- The test shall be performed by the external party as per their respective SOP, reviewed, and accepted by the plant. Refer the Attachment for SOP
-
- Reference SOP’s and results should be enclosed with the report.
-
- The formula to calculate the ACPH is as follows-
- Air changes per hour (ACPH) = Total CFM X 60
- Room volume
-
- Apparatus Required:- Anemometer, Capture hood. A calibrated instrument should be used for measurement.
-
- Acceptance criteria:- Air velocity and Air Change Per Hour (ACPH) shall be within the design specification. A variation in air volume shall not be ±20% from the design CFM
-
- Result:- Refer enclosed file named FORM-A of respective AHU requalification report. Traceability certificates should be enclosed along with the requalification report.
-
Integrity test Of HEPA Filter:
-
- The test shall be performed by the external parties as per their respective SOP, reviewed, and accepted by the plant. Refer the Attachment for SOP Reference SOP’s and results should be enclosed with the report.
-
- Apparatus Required:- Aerosol Photometer. A Calibrated instrument should be used for measurement.
-
- Acceptance Criteria:-The leakage rate is NMT 0.01%. Ensure zero (0 %) leakage at joints.
-
- Result:-
-
- Refer enclosed file named FORM-B of respective AHU requalification report.
-
- Traceability certificates to be enclosed along with the report.
-
- If any leakage is detected in the joints of filter it shall be repaired with the food-grade silicon and leak site shall be rescanned.
-
- Upstream concentration should be 100%.
-
Differential Pressure Measurement:-
- Differential pressure of the room shall be recorded using the calibrated instrument, once in two hours and it shall be continued for 72 hours.
-
- Acceptance criteria:- Pressure differentials should meet the requirement as specified in the system specifications.
-
- Result:- Record the differential pressure in the FORM-C of the requalification report.
-
Temperature And Humidity Control Test:-
-
- The air handling system shall be in operation for at least 15 minutes prior to performing this activity.
-
- In the case of manual recording, record the data for 72 hours at an interval of 2 hours frequency.
-
- If data loggers are using, record the date for a period of 72 hrs at 5 minutes interval.
-
- All lights in the critical and controlled areas should be ON during the testing.
-
- Observe the temperature and relative humidity use calibrated thermo hygrometer.
-
- Temperature and RH in the area shall be checked and recorded in dynamic conditions.
-
- Acceptance criteria:-
-
- Temperature and relative humidity should meet the requirement as specified in the system specifications. Refer point No. 6.1 system specification for predetermined acceptance criteria.
-
- Result:-
-
- Measure and record temperature and RH using sling psycho meter, thermo hygrometer, or data logger. Record the observations on FORM-D of the AHU requalification report.
-
Air Flow Pattern Test:-
-
- The test shall be performed by the external party as per their respective SOP, reviewed, and accepted by the plant.
-
- The test shall be performed in Rest as well as Dynamic condition.
-
- Reference SOP’s and results should be enclosed with the report.
-
- Video recording shall be performed for the airflow pattern test.
-
- Enclose the unedited CD of flow pattern along with the requalification report.
-
- Apparatus required:-
-
- Fog generator, water generated fog, and digital video camera.
-
- Acceptance criteria:-
-
- Air should flow from the higher-pressure zone to the lower pressure zone in the room to room.
-
- The air should flow unidirectionally from supply towards the return air filter or grill within the room.
-
- In videography show the exact area name and supply return grill’s ID.
-
- Videography shall be carried out to demonstrate the airflow pattern.
-
- Result recording:-
-
- Observe the airflow pattern as per the procedure mentioned above and record in the FORM-E in the requalification report.
-
Non-Viable Particle Count (NVPC) – HVAC Qualification Protocol:-
-
- The test shall be performed by the external party as per their respective SOP, reviewed, and accepted by the plant.
-
- Reference SOP’s and results should be enclosed with the report.
-
- The test shall be performed at rest and in operation both the condition.
-
- Attach the print out original and one photocopy of original with the qualification report and data shall also be recorded and compiled in the report.
-
- The no. of sample locations shall be calculated according to the below-mentioned table.
-
- Refer annexure No.: _________ for location details of area.
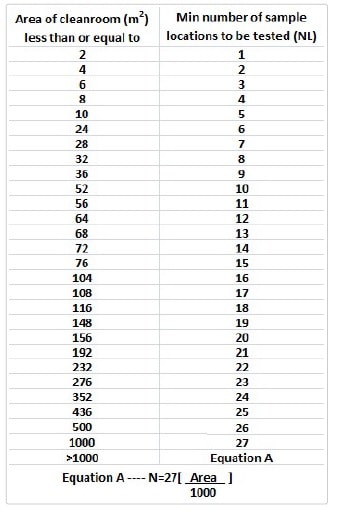
-
- Apparatus Required: Particulate Counter. A calibrated instrument should be used for measurement.
-
- Acceptance Criteria:- The average particle concentration at each of the particle measuring locations should fall the below-mentioned class limit.
| Class /Grade Size |
AT REST |
IN OPERATION |
|||
| Maximum number of permitted particles per cubic meter equal to or above | |||||
| 0.5 mm | 5.0 mm | 0.5 mm | 5.0 mm | ||
| A / 5 | 3,500 | 0 | 3,500 | 0 | |
| B / 6 | 3,500 | 0 | 3,50,000 | 2,000 | |
-
- Result Recording
-
- Data shall be collected in the datasheet.
-
- Measure and Record the particulate count at various locations as the attached sheet.
-
- The recording shall be done in FORM-F of the requalification report.
-
- Attach the calibration certificate of Particle Counter.
-
-
Recovery Study:
-
-
- The test shall be performed by the external parties as per their respective SOP.
-
- Reference SOP’s and results should be enclosed with the report.
-
- Attach the print outs, original and photocopy provided by the external agency of particle form of cleanroom from the initial stage of contaminated area till recovery.
-
- Ensure that the master instrument is calibrated and enclose the calibration certificate along with the requalification report.
-
- Recovery study should be performed for Temperature, RH, and Differential pressure.
-
- Record the readings and evaluate them with the design specification.
-
- Recording frequency should be 02-minute time interval by manual entries or using data loggers.
-
- Recovery test for viable counts should be checked on 15 minutes time interval 15 minutes, 30 minutes, 45 minutes, and 1 hour.
-
- Apparatus: Particulate Counter, Hygrometer, differential pressure gauge.
-
- Acceptance criteria: The recovery time should not be more than 15 minutes excluding viable count.
-
- Result Recording: Record the observations in FORM-G of the requalification report.
-
Microbial Monitoring:
-
- To determine the viable particle count test by exposing the settle plate and air sampling in the defined areas.
-
- The procedure shall be followed as per the respective SOP.
-
PASSIVE AIR SAMPLING:-
-
- Procedure:
-
- Plates shall be exposed on plate exposure stand at the pre-defined locations mentioned in individual format for each stream for not less than 4 hrs.
-
- Keep the plates on the upper platform of plate exposure stand, lift, and slide open the lid of the media plate and keep on the lower platform of the plate exposure stand.
-
- Exposed plates shall be recovered after 4 hrs. of exposure and incubate at specified conditions; SCDA plates: 20-25ºC for 72 hrs. Then 30-35ºC for 48 hrs.
-
- Plates shall be observed for any microbial growth after 5 days.
-
- Frequency:
-
- Microbial monitoring shall be done for three consecutive days once every six months.
-
- Acceptance criteria:
| Class / Grade | Limit (CFU/Plate) | Alert Limit | Action Limit |
| Grade A / 5 | <1 | <1 | <1 |
| Grade B / 6 | 5 | 3 | 4 |
| Grade C / 7 | 50 | 30 | 40 |
| Grade D / 8 | 100 | 60 | 80 |
-
- Result recording: Record the observations in FORM-H of the requalification report.
-
Active Air Sampling:
-
- Procedure :
-
- Battery of the Air Sampler shall be ensured for fully charged. Sterilized sieve shall be used during every monitoring exercise in case of an aseptic area.
-
- Active sites of the sampler shall be sanitized properly with filtered 70% IPA before each sampling.
-
- The pre-incubated media plate shall be placed after opening the lid. The perforated lid shall be placed above the plate & the sampler shall be switched on with feed parameter of 1000 liters of air.
-
- 1000 liters of the air shall be withdrawn.
-
- The plate shall be removed by taking care; not to touch the surface of the media & re-place the lid of the plate.
-
- After each sampling Air Sampler perforated lid shall be moped with 70% IPA.
-
- The plate shall be recovered after sampling and incubate at specified conditions; SCDA plates: 20-25ºC for 72 hrs. then 30-35ºC for 48 hrs.
-
- Frequency: Microbial monitoring shall be done for three consecutive days once every six months.
-
- Acceptance criteria:
| Area (Grade) | Limit (CFU/m3) | Alert Limit | Action Limit |
| A | <1 | <1 | <1 |
| B | 10 | 6 | 8 |
| C | 100 | 60 | 80 |
| D | 200 | 120 | 160 |
-
- Result recording: Record the observations in FORM I of the requalification report.
-
Re-qualification criteria:
-
- The system should be revalidated under the following conditions.
-
- If any major changes or modification in the system is done.
-
- Any major changes have been done in the respective room or module, which is affecting the environmental condition.
-
- If any major maintenance has taken place in the system which can affect the performance of the air handling system.
-
- Periodic requalification.
8.0 Deviation (If any):
-
- Any deviation observed during requalification shall be recorded and investigated.
-
-
- A) Description of deficiency and date observed.
-
-
-
- B) The person responsible for corrective action and date assigned.
-
-
-
- C) Corrective actions are taken and the date conducted.
-
9.0 Requalification Summary :
-
-
- The requalification report shall consist of a summary document, in narrative form, which shall briefly describe the activity performed along with the observations recorded in relevant exhibits.
-
-
-
- This report shall also include the related documents and attachments/annexures which were completed at the time of revalidation activity.
-
10.0 Approval of requalification Report:
-
- The report shall be evaluated and proper references/conclusions/recommendations shall be recorded by Quality Assurance.
-
- The report shall be evaluated and finally approved by Quality Assurance.
11.0 List Of Form/Annexure:
-
-
List Of Form:
-
| Form | Title |
| A | Air velocity/airflow measurement and calculation of air changes. |
| B | Filter integrity test. |
| C | Differential pressure measurement. |
| D | Temperature and humidity measurement. |
| E | Airflow pattern test |
| F | Non-viable particle count test. |
| G | Recovery |
| H | Viable particle count- Passive air sampling |
| I | Viable particle count- Active air sampling |
-
-
List of Annexure:
-
-
- Requalification report shall include the following annexure:-
| S. No. | Annexures |
| 01 |
Report of Air velocity and ACPH. |
| 02 |
Report of filter integrity. |
| 03 |
Report of Temperature and Relative Humidity. |
| 04 |
Report of Differential pressure. |
| 05 |
Report of Non-viable particle count. |
| 06 |
Environment Monitoring Report for Passive air sampling. |
| 07 |
Environment Monitoring Report for Active air sampling. |
| 08 |
Recovery study test report. |
| 09 |
CD of Airflow pattern test. |
| 10 |
Calibration certificate of Differential pressure gauge. |
| 11 |
Calibration certificate of data loggers. |
| 12 |
Calibration certificate of Anemometer. |
| 13 |
Calibration certificate of the aerosol photometer. |
| 14 |
Calibration certificate of a particle counter. |
| 15 |
Training Records. |
| 16 |
SOP of an external agency. |
| 17 |
Personal Monitoring report. |
| 18 |
Traceability Certificates |
12.0 References:
-
- SOP for qualification and requalification Of HVAC
13.0 Abbreviations:
| Abbreviation | Definition |
| AHU | Air Handling Unit |
| SOP | Standard Operating Procedure |
| RC | Regulatory Compliance |
| QA | Quality Assurance |
| ID | Identification |
| CFM | Cubic Feet per Minute |
| P | Protocol |
| m2 | Meter Square |
| HEPA | High-Efficiency Particulate Air |
| HVAC | Heating Ventilation and Air Conditioning |
| ISO | International Organization for Standardization |
| MOC | Material Of Construction |
| ACPH | Air Changes Per Hour |
| % | Percentage |
| QA | Quality Assurance |
| Temp. | Temperature |
| RH | Relative Humidity |
| WHO | World Health organization |
| DP | Differential Pressure |
| CD | Compact Disc |
| NVPC | Non-viable particle count |
| SCDA | Soya bean casein digest agar |
| NLT | Not Less than |
| NMT | Not more than |
| HOD | Head of Department |
| Ltd. | Limited |
| No | Number |
| Rev. | Revision |
| Sign. | Signature |
| A/L | Airlock |
| H.P. | Himachal Pradesh |
| µ | Micron |
| Cu. | Cubic |



Pingback: Technical Agreement - Preparation & Handling - Pharma Beginners