Process Validation: Establishing documented evidence through collection and evaluation of data from the process design stage to routine production, which establishes scientific evidence and provides a high degree of assurance that a process is capable of consistently yield products meeting pre-determined specifications and quality attributes.
SOP and Protocol for Process Validation of Drug Product
1.0 PURPOSE:
-
- The purpose of this procedure is to provide a high degree of assurance of meeting all the predefined attributes and the process is capable of consistently delivering a quality product.
2.0 SCOPE:
-
- This SOP is applicable for Process Validation / Qualification activities of drug products.
3.0 REFERENCES:
-
- In House
-
- Protocol & Report Numbering And Issuance System SOP.
-
- SOP for Change Control Procedure
-
- Planned Modification System SOP
4.0 RESPONSIBILITY:
-
- Quality Assurance Dept. shall responsible for preparation of process validation protocol, collection of process validation sample, and preparation of Process Validation report.
-
- Quality Control Dept. shall responsible for the analysis of the Process Validation sample and provide the data for the process validation report to Quality Assurance Dept.
-
- Maintenance Dept. shall responsible for preventive maintenance and calibration of equipment and instruments respectively.
-
- QA Head shall review & approved process validation protocol, approve validation report for its completeness and correctness with respect to all data and report, and to ensure implementation of SOP.
5.0 PROCEDURE
-
-
PROCESS Validation METHODOLOGY
-
This guidance describes the process validation activities in three stages:
-
-
Stage 1 – Process Design:
- The commercial process is defined during this stage based on knowledge gained through development and scale-up activities.
-
-
-
- The goal of this stage is to design a process suitable for routine commercial manufacturing that can consistently deliver a product that meets the majority of its quality attributes of activities related to stage -1 shall be performed, suggested by FDD.
-
-
-
Stage 2 – Process Validation:
- During this stage, the process design is confirmed as being capable of reproducible commercial manufacturing.
-
This stage shall be done in two parts:
-
Design of the facility and Qualification of the equipment and utilities:
-
-
-
-
- Qualification of utilities and equipment shall be covered under individual plans or as part of an overall project plan.
-
-
-
- The details of the same shall be mention in the Protocol.
-
-
-
- Qualification activities must be completed prior to the start-up of the Process Performance Qualification (PPQ) stage.
-
-
-
- The suitability of equipment and utilities must be documented in accordance with the process requirements in all the anticipated operating ranges.
-
-
-
-
Process Performance Qualification:
-
-
-
-
- During stage 2 and onward, cGMP compliance must be followed.
-
-
-
- Successful completion of stage 2 is necessary before commercial distribution.
-
-
-
- Need of training shall be assessed prior to the start-up of PPQ batches.
-
-
-
- During this stage, the process design is evaluated to determine if the process is capable of consistently manufacturing the product meeting predetermined acceptance criteria.
-
-
-
- Process for new as well as existing products shall be qualified.
-
-
-
- Process qualification shall run according to approved protocol detailing sampling, timing, location, procedures alongwith analytical tests and acceptance criteria.
-
-
-
- Three batches of commercial batch size shall be taken for qualification in accordance to the Process Qualification protocol and BMR.
-
Stage 3 – Continued Process Verification:
- Ongoing assurance is gained during routine production that the process remains in a state of control.
-
-
-
- During this stage, continuous monitoring of process parameters and quality attributes at the level established during the process validation stage shall be done.
-
-
-
- This stage is applicable for Existing Products, Site Transfer Products, and New Products.
-
-
-
Process Development and Trial Batch Plan:
- R&D/FDD shall generate knowledge and understanding about the manufacturing process and the product at the development stage.
-
-
-
- R&D shall issue a MPS to the manufacturing site defining
- Manufacturing process,
- R&D shall issue a MPS to the manufacturing site defining
-
-
-
-
- Critical process parameters,
-
-
-
-
-
- In-process checks,
-
-
-
-
-
- Specifications for input materials,
-
-
-
-
-
- Intermediate products and
-
-
-
-
-
- Final products.
-
-
-
-
- Based on the requirement and risk assessment R&D shall recommend for the trial batch(es) manufacturing prior to commercialization.
-
-
-
- The batch/lot size of the trial batch shall be decided based on the equipment occupancy level and other scientific rationales so that the data, observation & experience from the trial batch will be useful for preparing the batch record and process validation protocol/report for commercial batches.
-
-
-
- The trial batch/lot size shall not be less then 1/10th of the intended commercial batch size, keeping the set of equipment same.
-
-
-
- Principle of operation shall be identical.
-
-
-
- Prepare a trial batch report as per the Annexure 4.
-
-
-
- The trial batch report shall be duly signed by the Production, QA, R&D, Manufacturing head and Quality head and shall be retained with QA for reference.
-
-
-
- Photocopy may attached to the relevant BMR.
-
-
-
- Based on the trial batch report & recommendations, Prepare the commercial batch manufacturing record & process validation protocol and Initiate the commercial batch manufacturing.
-
-
-
- R&D shall revise and send the MPS to the site prior to post validation BMR revision, if any revision is recommended /identify during execution of process validation batches.
-
-
-
Process Validation Pre-Requisites:
-
Prior to Process Validation study. Complete the following prerequisite activities .
-
-
- Qualify the Manufacturing Equipment and utilities to meet cGMP requirements.
-
-
-
- Qualification of Utilities to be used in manufacture of the product (example – purified water, compressed air, HVAC system etc.) .
-
-
-
- Calibrate the Instruments used in processing (example – weighing balance. vernier calipers, hardness tester etc.).
-
-
-
- Validate the Analytical methods for in process testing and finished product analysis .
-
-
-
- Train appropriately the personnel involved in manufacturing and testing of process validation batches .
-
-
-
Process Validation Protocol:
-
On satisfactory completion of pre requisite activities, Prepare the process validation protocol as described below.
-
-
- QA shall prepare the Process Validation protocol as per Annexure-3.
-
-
-
- This protocol shall applicable for both commercial as well as trial batches.
-
-
-
-
In case of process validation protocol of clinical trial batches,
- FDD shall review the Protocol / report and approve prior to execution at site,
-
-
-
-
-
- Approval of protocol can ensure by additional signature on same protocol as a proof or.
-
-
-
-
-
- Attach any supporting communication to the respective clinical trial batch process validation protocol.
-
-
-
-
- Production and QC shall review the Process Validation Protocol.
-
-
-
- Head QA shall approve the protocol.
-
-
-
- The Process Validation team members of different departments (Production, QC& QA) shall review Process Validation Protocol for the correctness of their relevant matters.
-
-
-
- Designated person from Production shall ensure the suitability of the equipments listed in the protocol;
-
-
-
-
- Whether the range and set point of process parameters is in line with measuring device available on the respective equipment / instrument;
-
-
-
-
-
- Whether the equipment and measuring instruments are in calibrated status.
-
-
-
-
- Designated person from QC shall verify
- The correctness of carried QC tests at different process stages and availability of required testing methodology .
- Designated person from QC shall verify
-
-
-
- Site validation coordinator or designee shall ensure the correctness of the
- Process description,
- Site validation coordinator or designee shall ensure the correctness of the
-
-
-
-
- Flow chart,
-
-
-
-
-
- Critical process parameters, their range and set point,
-
-
-
-
-
- Input material, their quantities,
-
-
-
-
-
- Names of vendors stated in the protocol.
-
-
-
-
- Designated person from QA shall verify whether
- Objective,
- Designated person from QA shall verify whether
-
-
-
-
- Scope,
-
-
-
-
-
- Process description,
-
-
-
-
-
- Process flow,
-
-
-
-
-
- List of equipment, equipment with qualification status,
-
-
-
-
-
- List of input materials,
-
-
-
-
-
- Critical process parameters,
-
-
-
-
-
- In-process checks are correct;
-
-
-
-
-
- Sampling plan is adequate to assess the capability of the process to consistently produce product meeting required specifications.
-
-
-
-
Execution of Process Validation:
- Execute a minimum of three consecutive batches against the approved BMR and the Process validation protocol.
-
-
-
- Where multiple batch sizes are available for a product, Perform the PV for each batch size.
-
-
-
- However PV plan can restrict to only those unit processes that are evaluated to have impact due to difference in batch size.
-
-
-
- For example if there is no change in lot size at Granulation stage and only number of lots increased,
-
-
-
- Perform the PV of only Blending operation and decide the extent of validation study of other stages based on the risk/impact assessment.
-
-
-
-
Where a product is manufactured in multiple strengths using a common blend,
- Employ a matrix approach for PV.
-
-
-
-
-
- For example if a product is manufactured as 10 mg, 20 mg and 40 mg by a common blend,
-
-
Then the PV can include validation up to blend stage with three batches of common blend and validation of subsequent unit processes like compression, coating etc. with three batches each strength.
-
-
-
- In such cases number of batches of different strength may reduce with appropriate justification and necessary approval from Customer / Regulatory agency.
-
-
-
-
- Collect the samples as per sampling plan defined in the PV protocol & tested in QC and PV team shall obtain the results to compiled for evaluation by the PV team.
-
-
-
- Capture the values of critical process parameters noted during in-process of the PV Batches as per Annexure-5 (applicable for both commercial as well as trial batches)
-
-
-
- The variations in the critical process parameters in lot to lot/batch to batch shall justify with scientific logic and shall capture in batch manufacturing record as well as PV.
-
-
-
-
At compression stage Challenge study performs like
- Hopper study,
-
-
-
-
-
- Speed study and
-
-
-
-
-
- Cycle study ranges shall perform for the minimum, optimum and maximum ranges and Record in the attachment of respective batch number.
-
-
-
-
- Checking of results from testing of in-process samples, intermediate product and final product of the PV Batches by QC person for correctness and compliance to respective acceptance criteria.
-
-
-
- Same shall checked by QA person independently.
-
-
-
- Variability ‘within’ a validation batch shall assess by QA by comparing the results of samples drawn from various locations / different intervals using the Relative Standard Deviation criteria pre-defined in the protocol.
-
-
-
- Likewise, QA shall assess the variability ‘between’ Validation Batches by comparing the process parameters and test results of each batch at every stage of testing with the other PV Results.
-
-
-
- Investigate significantly different to determine the cause of variability.
-
-
-
Process Validation Report
- QA shall prepare the process validation report by compilation of BMR data and QC analytical report as per Annexure 4
-
-
-
- Record all data during execution of process validation batches as per Annexure 5 for each batch.
-
-
-
- Preparation of the interim report first, second and third after completion of manufacturing and packing process of respective batches.
-
-
-
- QA shall prepare the interim report and reviewed by Production department and approved by QA Head.
-
-
-
- Compile all finished product result as per acceptance criteria and Attach with reports.
-
-
-
- Any change control/events observed during processing of PV batches shall handle as per Change control procedure and event SOP respectively.
-
-
-
- Prepare a process validation summary report after completion of PV batches as per Annexure 6.
-
-
-
- QA shall prepare Process validation summary report and reviewed by Production and approved by QA Head.
-
-
-
- Share the approved Process Validation summary report with production department to freeze all the critical process parameters and revise the BMR.
-
-
-
Release of Process Validation batches for distribution
- Release the PV batches for distribution after:
- Successful completion of PV activity and review, approval and signing off the PV interim report with supporting raw data.
- Release the PV batches for distribution after:
-
-
-
- Ensure no impact on product quality prior to release of each PV batch.
-
-
-
Re-Process Validation criteria:
- In case of any change in facility.
-
-
-
- In case of any change in manufacturing equipment.
-
-
-
- Change in vendor of Active Pharmaceutical Ingredient
-
-
-
- Change in batch size
-
-
-
- Based on associated risk and impact analysis the extent of PV shall decide which may include the entire process that is impacted.
-
-
-
For introduction of a New product in the facility:
- Initiate a permanent change control by the user department and approved as per SOP for Change Control Process.
-
-
-
- After assessment of all the possible impacts. Initiate the manufacturing of PV batch along with simultaneously the risk assessment report.
-
-
-
- Take initial three consecutive batches of new product for PV.
-
-
-
- Identify all the critical process parameters in the protocol for the particular product and Manufacture the batch by referring the tentative limit as provided in MPS.
-
Note:-
-
-
- Consider the tentative limits of critical process parameter and their control limit mentioned in the MPS .
-
-
-
- Record if any deviation observed during the manufacturing process in the respective BMR.
-
-
-
- QA/ FDD representatives shall verify such deviations and write the appropriate remark in the concern page of BMR.
-
-
-
- In case more parameters of any stage needs to established. Attache an addendum to the concern pages with sign and date of Production, QA and FDD representatives.
-
-
-
- Establish parameters which are indicative and during PV shall established /freezed after successful completion of PV
-
-
-
- The actual reading obtained during wet granulation is likely to vary from the limit mentioned from the MPS.
-
-
-
- Similarly the limits provided in MPS for Hardness/thickness/ yields are indicative only and need to establish during PV.
-
-
-
- Establish all identified Tentative/ indicative established parameters highlighted by following Good Documentation Practices like application of Symbols and putting remarks against the symbol on the same page.
-
-
-
- Perform the challenge study during process validation batches, which shall include Full Hopper, Low Hopper, Low RPM, High RPM, Low Hardness, High Hardness and .
-
-
-
- Perform the impact of challenge study for minimum 30 minutes or based on risk assessment, studied on final product.
-
-
-
-
Update the processing parameter based on findings.
-
-
-
-
- Perform the challenge study at the start of the compression operation after initial machine setting verified by QA.
-
-
-
- QA shall prepare the protocol for PV and carryout sampling and testing of physical parameter as per the approved protocol.
-
-
-
- QA shall verify the parameter and record in the protocol/BMR.
-
-
-
- To monitor and record the NRR details at various stages (Granulation & Compression) of manufacturing in case of Bilayer Tablet as per Annexure 7.
-
-
-
- Requirement of any of the PV batches shall decided by FDD/QC/QA and shall part of PPQ.
-
-
-
- Based on product, process, technical criticality, Adopt the reduced sampling plan and Mention the details in the sampling plan of respective protocol.
-
-
-
- Collect the sample for chemical analysis and sent to QC by QA.
-
-
-
- After completion of the analysis, QC shall submit the analytical reports to QA and QA shall prepare the PV report.
-
-
-
- QA Head and Quality Head shall approve the Process Validation report.
-
-
-
- Revise the BMR and BPR after completion of process validation report.
-
-
-
- QA shall maintain status of process validation batches of new product and existing product as per given Annexure 2.
-
6.0 ABBREVIATIONS:
-
-
- cGMP : current Good Manufacturing Practices
-
-
-
- FDD : Formulation and Development Department
-
-
-
- HVAC : Heating Ventilation and Air Conditioning
-
-
-
- MPS : Master Product Specification
-
-
-
- PMS : Planned Modification System
-
-
-
- PPQ : Process Performance Qualification
-
-
-
- PVMP : Process Validation Master Plan
-
-
-
- RPM : Rotation per minute
-
-
-
- R&D : Research and Development
-
-
-
- SOP : Standard Operating Procedure
-
-
-
- VMP : Validation Master Plan
-
7.0 DEFINITION:
-
-
Manufacturing process:
- Transformation of starting materials into finished products through a single operation or a sequence of operations involving processing equipment, environmental control, personnel and documentation.
-
Validation:
- Validation is the documented act of proving that
-
-
-
- Any procedure,
-
-
-
- Process,
-
-
-
- Equipment,
-
-
-
- Material,
-
-
-
- Activity or system actually leads to the expected result.
-
Product life cycle:
- Stages through which a product moves from its inception till its discontinuation. It includes pharmaceutical development. technology transfer and commercial production up to product discontinuation.
-
8.0 ANNEXURES:
Flow Chart of Validation Approach (Annexure 1)
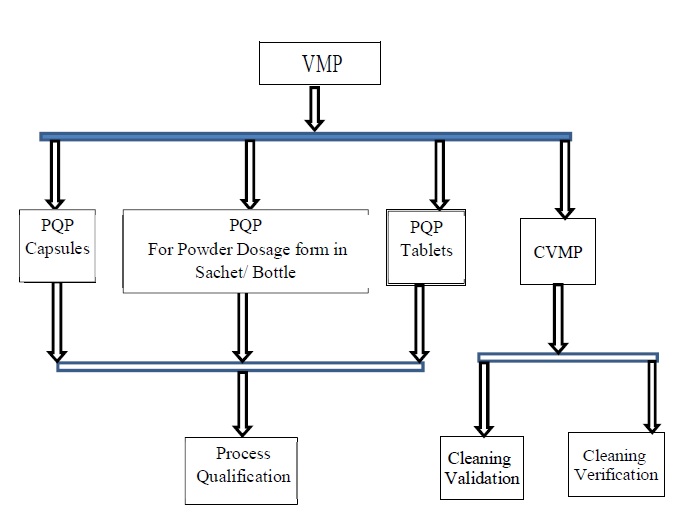
-
-
Status of Process Validation Batches (Annexure 2)
| Sr. No. | Product Name | Protocol No. | Report No. | Effective Date | Batch Size | No. of Batches | Batch No. | Remark |
| 1st | ||||||||
| 2nd | ||||||||
| 3rd |
Protocol Template (Annexure 3)
Cover Page :
| Product | |
| Dosage Form | |
| Label Claim | |
| Therapeutic Category | |
| Product Code | |
| Shelf life | |
| Batch Size | |
| Packing Profile | |
| Supersede Protocol No. | |
| Protocol approved on | |
| Effective Date |
Note: This protocol can be customized as per the product, process, technology involved in the processes of any product.
- Table of Contents
| Sr. No. | Contents | Page No. |
| 1 | ||
| 2 | ||
| 3 |
- Purpose
- The purpose of this protocol is to establish documented evidence, which will provide a high degree of assurance that the adopted manufacturing process methodology for the product ………………… is capable of providing consistent and reproducible result as per the pre-defined specification & its quality characteristics/attributes
- Scope
- This protocol is applicable for Process Qualification of …………, batch size …….. Lac /…………..Kg to be manufactured at _____________________________________;
- Responsibility
-
-
Quality Assurance Department
-
-
-
- Prepare, review, approve and execution of protocol.
- Provide training to concerned personnel.
- Withdraw the samples as per the sampling plan.
- Monitor validation activities.
- Review the validation data, and
- Provide the final conclusion of the Process qualification in the reports
-
Production Department
- Involve trained personnel in manufacturing activities.
- Perform processing of the batch as per the batch manufacturing records.
- Ensure that qualified equipment’s are used.
- Assist Quality Assurance department in withdrawal of sample.
- Review process qualification protocol
-
Quality Control Department
- Perform testing and analysis in support of the validation activity.
- Generate and compile the analysis data.
- Review the process qualification protocol.
-
Maintenance Department
- Ensure that all processing equipment and instruments are in a state of qualified and calibrated.
- Provide proper utility services during the processing of qualification batches.
-
5.0 Training Record
-
- Training shall be imparted to all concerned personnel up to the operator level involved prior to execution of this protocol.
| Purpose | To train all personnel involved in the execution of this qualification protocol for following topics. | ||
| Topic | Purpose
Sampling and acceptance criteria Documentation |
||
| Trainer | |||
| Name of the participant | Area of operation | Signature/Date | |
6.0 Abbreviations
-
- _____________________________________________________________________
- _____________________________________________________________________
7.0 Validation Approach
-
- Product ……………….. (Each Film coated tablets contain ………………………… mg) is a new product at this location, the batch size is determined ……… lacs /……… kg, this product will be manufactured in granulation area….. This Product contains step like …………………………
-
- Thus to validate the manufacturing process, three consecutive batches will be considered and sample shall be collected at appropriate stage as per sampling plan. The equipment set will be remained identical for all three validation batches.
8.0 Process flow chart
| Raw materials | Process | Equipment |
9.0 List and quantity of Raw Materials ( Batch Size ………Lacs / ……. kg)
10.0 List and quantity of Packing Materials ( Batch Size …… lacs)
11.0 List of Equipment’s used in Manufacturing & Packing and Its Qualification Status
|
Sr. No. |
Equipment | Equipment ID No. | Make | Capacity |
Qualification Status |
|
| Date of Qualification |
Due Date |
|||||
| 1 | Vibro Sifter | |||||
| 2 | Clit Mill | |||||
| 3 | RMG | |||||
| 4 | FBD | |||||
| 5 | Blender | |||||
| 6 | Bunker | |||||
| 7 | Compression Machine | |||||
| 8 | Coating | |||||
| 9 | Blister | |||||
12.0 Key Process Parameter and its Control
-
- Following key parameters are required to be validated for the manufacturing of Product Name
|
Sr. No. |
Process step | Process Parameter to be validated |
Recommendation |
||
| 1. | Sifting & Milling | Sieve Size | |||
| Screen size | |||||
| 2. | Dry Mixing | Impeller/chopper Speed* | |||
| Mixing Time* | |||||
| 3. | Wet Granulation | Binder addition time | |||
| Ampere load. | |||||
| 4. | Drying | Inlet Temperature* | |||
| Outlet Temperature* | |||||
| LOD (@105°C for 5 min.) | |||||
| Total Drying Time | |||||
| 5. | Dry Milling & Sifting | Screen Size | |||
| Sieve Size | |||||
| 6. | Lubrication | Lubricant sifting | |||
| RPM of Blender | |||||
| Mixing Time | |||||
| 7. | Compression | Compaction Force | |||
| Turret RPM | |||||
| 8. | Coating | Inlet Temperature | |||
| Outlet Temperature | |||||
| Spray rate | |||||
| Pan RPM | |||||
| 9. | Packing | Temperature of Sealing roller* | |||
| Temperature of Forming roller* | |||||
| Machine Speed | |||||
13.0 Sampling Plan and Acceptance Criteria
| A) Physical Parameter | |||||
| Sr.
No. |
Stage | Test parameter | Number of Location
|
Sample Quantity | Acceptance
Criteria |
| 1 | Drying | LOD | T1, T2, M1, M2, B1 & B2.
(One Composite sample) |
1-3 gm each sample & LOD perform on composite sample
(3.0 gm) |
For Information Only |
| 2 | Lubrication | Bulk density
Tapped density Hausner ratio Carr index Sieve Analysis |
Top, Middle &
Bottom (One Composite Sample) |
200 gm
|
For Information Only |
| 3 | Compression
A)Hopper study |
Weight variation
Hardness Thickness |
Full Hopper, Half Hopper & Low Hopper.
(LHS/RHS) |
No. of stations+5 Tablets/sample
|
As Per BMR |
| B) Speed study | Weight variation
Hardness Thickness Friability Disintegration |
(High speed, Optimum speed & Low speed
(LHS/RHS) |
…..Tablets/sample
(No. of stations+5 Tablets for Wt. Variation, Hardness & Thickness …Tablets for friability 06Tablets for DT) |
As Per BMR |
|
| C) Cycle study | Weight variation
Hardness Thickness Friability Disintegration |
Initial, Middle & Final (LHS / RHS) | …..Tablets/sample
(No. of stations+5 Tablets for Wt. Variation, Hardness & Thickness …Tablets for friability 06 Tablets for DT) |
As Per BMR |
|
| B) Chemical analysis | |||||
| Sr.
No. |
Stage | Test parameter | Number of Location
|
Sample Quantity | Acceptance
Criteria |
| 1 | Lubrication | Description
Blend Uniformity |
10 Location.
(T1, T2, T3, M1, M2 M3, B1, B2, B3 & LB). |
10 Samples In triplicate
( Total 30 Samples). …… mg/sample |
As per Specification |
| 2 | Compression | Assay | Initial, Middle & End Stage | 3 Samples
20 Tablets/Sample |
As per Specification |
| 3 | Coating | Complete Analysis | Composite sample
(Top, Middle & Corner) |
100 Tablets | As per finished product specification |
14.0 Selection of batches
-
- Three consecutive batches shall be selected for process qualification having same / identified set of equipment
15.0 Deviations/Incidents
-
- If any deviation or incident observed in the process qualification batches shall be discussed and resolved as per SOP and shall be recorded in the process qualification report.
16.0 Change Control Management
-
- If any change observed in the process qualification batches shall be allowed only through Change control Management procedure and shall be recorded in the process qualification report.
- Change control not required for indicative parameter.
17.0 Re-validation criteria
-
- Change in Manufacturing Process
- Process Parameter changes
- Change in make, model or capacity of the equipment
- Batch size change
- Change in the Vendor of API& excipients
- Every five years if no change in process, equipment’s, raw materials and composition
18.0 Validation Report
-
- Validation report shall be prepared by compiling the data obtained from three consecutive batches and a conclusion shall be drawn.
- The data generated during the qualification activity shall be attached with the process validation report.
19.0 Diagram of RMG & Occupancy of material in RMG
20.0 Diagram of FBD Bowl & Bunker
21.0 Diagram of Hopper
22.0 Approval of Protocol
-
- This protocol has been studied for adequacy and approved by the following responsible personnel
|
Name |
Signature/ Date |
|
| Prepared By:
(Quality Assurance) |
||
| Reviewed By:
(Production/FDD) |
||
| Reviewed By:
(Head, Quality Control) |
||
| Reviewed By:
(Head, Quality Assurance) |
||
| Approved By:
(Site Quality Head) |
Report Template (Annexure 4)
-
- Refer above (Annexure 3 ) with filled data
In process Sheet (Annexure 5)
Attachment of In process sheet – click to Download
- Also visit : Technology Transfer of Drug Product



Pingback: Continued Process Verification Guideline & SOP - Pharma Beginners
Pingback: SOP for Drug Product Recall & Mock Recall - Pharma Beginners
Pingback: SOP for Protocol and Report Numbering System - Pharma Beginners
Pingback: Cleaning Validation master plan (CVMP)-New Approach - Pharma Beginners
Pingback: Document Management System in Pharmaceuticals - Pharma Beginners