Data integrity is a fundamental pillar in the field of pharmaceuticals, playing a crucial role in maintaining the quality and safety of medicinal products. In an industry where human lives are at stake, the accuracy and reliability of data are of utmost importance. Therefore, it is imperative for pharmaceutical companies to establish robust systems and practices to ensure data integrity throughout the entire product life cycle.
Ensuring Data Integrity in Pharmaceuticals
Data integrity refers to the completeness, accuracy, and consistency of data, ensuring that it remains unaltered and reliable. It encompasses various aspects, including data collection, processing, storage, and retrieval. By maintaining data integrity, pharmaceutical companies can uphold the highest standards of quality, efficacy, and safety in their products.
SOP : Handling Data Integrity Observations (DIO)
One of the primary reasons data integrity is crucial in the pharmaceutical industry is to prevent errors and inaccuracies that may lead to adverse effects on patients. Inaccurate data can result in incorrect formulation, improper dosage, or even the presence of impurities in medicines. Such errors can have severe consequences, ranging from ineffective treatment to life-threatening complications. Therefore, pharmaceutical companies must implement stringent measures to guarantee the integrity of data used in the development, manufacturing, and distribution of medicines.
Furthermore, data integrity is essential for regulatory compliance.
Regulatory bodies, such as the Food and Drug Administration (FDA), require pharmaceutical companies to maintain accurate and reliable data to ensure the safety and efficacy of their products. Non-compliance with data integrity regulations can lead to severe penalties, including product recalls, fines, and damage to a company’s reputation. Therefore, adherence to data integrity guidelines is not only a legal obligation but also a crucial aspect of maintaining a pharmaceutical company’s credibility and trustworthiness.
To ensure data integrity, pharmaceutical companies must implement comprehensive quality management systems. These systems should include robust documentation practices, standardized operating procedures, and regular audits. Additionally, companies should invest in advanced technologies and secure data storage systems to minimize the risk of data manipulation or unauthorized access. Regular training and education programs for employees are also essential to create a culture of data integrity and awareness within the organization.
In conclusion, data integrity is a vital aspect of pharmaceutical quality. It is essential for ensuring the accuracy, reliability, and consistency of data throughout the product lifecycle. By maintaining data integrity, pharmaceutical companies can safeguard the health and well-being of patients, comply with regulatory requirements, and uphold their reputation in the industry. Therefore, it is imperative for companies to prioritize data integrity and establish robust systems and practices to guarantee the highest standards of pharmaceutical quality system.
1.0 What is “data integrity” in pharmaceuticals?
Data Integrity is critical to pharmaceutical organizations because it drives critical processes like manufacturing, regulatory compliance, clinical trials, and drug development. Continuous Data integrity preserves belief in the safety, efficacy, and quality of pharmaceuticals.
In today’s environment, it is critical to understand the “Data Life cycle” from the point of beginning to the final report. The key to making sensible choices lies in maintaining the integrity of data, from its collection to its reporting. Data forms the basis for important decisions, and data with integrity gives these decisions accuracy and dependability. Adopting digital data and its governance has benefits that enable quicker and more precise decision-making. Following strong guidelines like ALCOA+ and abiding by legally binding regulations like the FDA’s 21 CFR 11, which are supported by extensive validation,
2.0 What is “metadata”?
Data about data, or metadata, consists of information about a piece of data that has been divided into highly structured fields and is mostly used for tracking, categorization, and analysis. It includes information about a data asset’s origin, history, versions, and other characteristics.
Information about data, such as its relationships, structure, format, and significance, is referred to as metadata. Pharmaceutical companies can find problems with data quality, learn more about how their data is used, and enhance their data integration procedures by collecting and evaluating metadata.
Rather than being an original concept, metadata is a recent addition to the list of elements which make up a “Complete” data record.
Common sense would be an easy rule for everyone. Have a third party who is not involved in the process examine the documentation if you have any doubts about it. You’ve passed the metadata test if they understand what’s there.
3.0 What does the FDA mean when it refers to record formats as “static” and “dynamic”?
Health authorities have issued guideline materials to protect the reliability and integrity of good manufacturing practice (GMP) records since data integrity is still a key concern for regulated pharmaceutical laboratories (1–5). GMP records and data are referred to as either static or dynamic in several sections of the guidance (2–5). As demonstrated in other data integrity articles, the phrases “static” and “dynamic” data are often used (6–8).
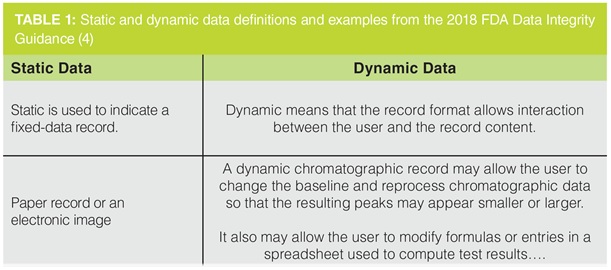
Identifying Dynamic and Static Data
Definitions of static and dynamic data are shown in Table 1, along with examples taken from FDA Data Integrity Guidance (4)’s question 1(d). According to the definition, static data is information that is unchangeable during the record retention term and seems to be unchanged once it is created. On the other hand, an analyst must evaluate dynamic data in order to provide a reportable outcome. Table 1 shows that spreadsheets and chromatography data are examples of dynamic data, while balance printouts are an example of static data.
4.0 What does a “audit trail” mean?
An audit trail is described as “a secure, computer-generated, time-stamped electronic record that allows reconstruction of the course of events relating to the creation, modification, and deletion of an electronic record” in the FDA’s guidelines for industry on computerized systems used in clinical trials.
The US FDA has a law called 21 CFR Part 11 that lays out the requirements for digital signatures and electronic documents. This legislation requires an audit trail that ensures traceability for all electronically stored records.
Since audit trails offer a record of all system actions and enable event reconstruction in the event that an investigation is necessary, they are an essential part of complying with 21 CFR Part 11 standards.
5.0 In § 211.68(b), how is the term “backup” used by the FDA?.
Backup: “a true copy of the original data that is maintained securely throughout the records retention period (for example, § 211.180)” is defined as backup in § 211.68(b) of the law. “People, machines, and methods organized to accomplish a set of specific functions” is how § 211.68 refers to systems.
6.0 In § 211.68, “computer or related systems,” what are the “systems”?
211.68 Electronic, mechanical, and automated apparatus. Drug products may be manufactured, processed, packed, and stored using automated, mechanical, electronic, or other forms of equipment, including computers, or related systems that will fulfill a function satisfactorily.
7.0 When can CGMP data be excluded from decision-making processes?
The FDA mandates that all data “maintained for CGMP purposes (e.g., § 211.180) and evaluated by the quality unit as part of release criteria (see §§ 211.22 and 212.70) must be created as part of a CGMP record.”” Data may only be excluded from consideration when determining release criteria with the provision of a sound, verified, and scientific rationale.
8.0 Does our computer system require validation for every workflow?
To put it simply, sure. According to the FDA, “a workflow is an intended use of a computer system to be checked through validation, such as creation of an electronic master production and control record (MPCR).” The FDA continues by saying that “you cannot know if your workflow runs correctly if you validate the computer system but do not validate it for its intended use.”
9.0 How should cGMP computer system access be limited?
“A unique individual cannot be identified through the login when login credentials are shared, and the system would thus not conform to the CGMP requirements in parts 211 and 212,” the FDA explains. The FDA requires that all system controls be created in a way that complies with all cGMP regulations in order to guarantee product quality.
The FDA advises the pharmaceutical industry to, whenever feasible, limit access to technical means for changing specifications, process parameters, or manufacturing or testing procedures (e.g., by limiting permissions to change settings or data).
10. How should blank forms be controlled?
Blank forms should be controlled by limiting their availability to only those who need them and ensuring that they are stored securely when not in use. Additionally, it is important to keep track of who has access to the blank forms and to monitor how they are used. The forms should be securely destroyed when no longer needed.
11.0 How often should audit trails be reviewed?
Audit trails should be reviewed regularly, at least on a monthly basis. Depending on the nature of the activities being monitored, more frequent reviews may be necessary. Additionally, reviews should be conducted after any significant changes or updates to the system or process.
12.0 Who should review audit trails?
Audit trails should be reviewed by an independent, qualified, and experienced auditor. The auditor should have the knowledge and expertise necessary to review the audit trail and should not be involved with the activities they are auditing.
13.0 Can electronic copies be used as accurate reproductions of paper or electronic records?
Yes, electronic copies can be used as accurate reproductions of paper or electronic records. This is because digital copies are exact replicas of the original document and can be used to verify the accuracy of the original. However, it is important to ensure that the digital copy is a true and accurate representation of the original document, as any errors or omissions in the digital copy could lead to inaccurate records.
14.0 Is it acceptable to keep static records or paper printouts from standalone computerized laboratory instruments, like an FT-IR device, rather than the original electronic records?
No, it is not acceptable to retain paper printouts or static records instead of original electronic records from stand-alone computerized laboratory instruments, such as an FT-IR instrument. Electronic records are more reliable and accurate than paper printouts, and they also provide a more efficient way to store and access data.
15.0 Can electronic signatures be used instead of handwritten signatures for master production and control records?
Yes, electronic signatures can be used instead of handwritten signatures for master production and control records. In most cases, electronic signatures are legally binding and allow for a secure and convenient way to verify information and documents. However, it is important to check the local laws and regulations before using electronic signatures to ensure compliance.
16.0 When does electronic data become a cGMP record?
In order for electronic data to be recognized and accepted by regulatory bodies, auditors, or individuals involved in Current Good Manufacturing Practice (cGMP) compliance, it must be produced or generated in a language that is widely accepted. Once it is translated into English, it becomes an official CGMP record. It is generally expected that the data be in English or translated into English to be considered a CGMP record.
17.0 Why has the FDA cited use of actual samples during “system suitability” or test, prep, or equilibration runs in warning letters?
The FDA cites the use of actual samples during these runs in warning letters to ensure that the testing methods and equipment used by pharmaceutical companies are reliable and consistent. By using actual samples, the FDA can assess if the system is suitable for accurate testing and if the results obtained are valid and reproducible.
The FDA cites the use of actual samples in warning letters to address concerns about the integrity and accuracy of pharmaceutical testing processes. By using actual samples, the FDA can evaluate if the system is properly calibrated, if the equipment is functioning correctly, and if the testing methods are suitable for ensuring the quality and safety of pharmaceutical products. This citation serves as a means to enforce compliance with regulatory standards and to encourage pharmaceutical companies to maintain rigorous quality control measures.
18.0 Is it acceptable to only save the final results from reprocessed laboratory chromatography?
The acceptability of only saving the final results from reprocessed laboratory chromatography depends on the specific requirements and standards of the scientific community or regulatory bodies involved. It is generally recommended to retain all relevant data and documentation, including raw data and intermediate results, to ensure transparency, reproducibility, and traceability of the analytical process. However, if the final results can be accurately and reliably traced back to the original data and if this practice aligns with the established guidelines and regulations, it may be deemed acceptable in certain cases. It is essential to consult the relevant authorities or experts in the field to determine the acceptability of this approach.
Saving only the final results from reprocessed laboratory chromatography without retaining the raw data and intermediate results may have several implications. Firstly, it can hinder the ability to verify the accuracy, reliability, and reproducibility of the analytical process. Raw data and intermediate results are crucial for conducting audits, troubleshooting, and conducting further analysis if needed. Secondly, this practice may raise concerns about data integrity and transparency, as it becomes difficult to trace and validate the final results. Thirdly, regulatory bodies or scientific communities may have specific guidelines or requirements regarding data retention, and failing to comply with these standards could lead to non-compliance issues or legal consequences. It is important to carefully consider these implications and consult relevant guidelines or experts before deciding to only save the final results from reprocessed laboratory chromatography
19.0 Can an internal tip regarding a quality issue, such as potential data falsification, be handled informally outside of the documented cGMP quality system?
No, it is not acceptable to handle such a tip informally outside of the documented cGMP quality system. The cGMP quality system is designed to ensure compliance with regulations and maintain the integrity of data. Any potential quality issues, including data falsification, must be handled through the established procedures outlined in the cGMP quality system to ensure proper investigation, documentation, and resolution.
Handling an internal tip about potential data falsification informally outside of the documented CGMP quality system can have serious consequences. It may lead to a lack of proper investigation, documentation, and resolution of the issue, compromising the integrity of data and potentially violating regulatory requirements. This can result in regulatory non-compliance, legal consequences, damage to the company’s reputation, and potential harm to public health and safety. It is crucial to follow the established procedures within the CGMP quality system to address such quality issues appropriately


