Standard Operating Procedure (SOP) for maintenance of laboratory instrument i.e. preventive maintenance, breakdown maintenance, etc.
SOP for Maintenance of Laboratory Instrument
1.0 Purpose:
-
- The purpose of this SOP (Standard Operating Procedure) is to describe the procedure of instrument maintenance and preventive maintenance.
2.0 Scope:
-
- This procedure is applicable to the Instruments at Quality Control laboratory in the pharmaceutical manufacturing plant.
3.0 References, Attachments and Annexure :

-
-
References :
- In-house procedure
-
-
- SOP for Breakdown Maintenance Procedure
-
Attachments :
- Under Maintenance Label………………………… (Attachments 1)
-
- Under Preventive Maintenance Label………….(Attachments 2)
-
- Preventive Maintenance History card………… (Attachments 3)
-
- Instrument History Card……………………………(Attachments 4)
-
- Preventive Maintenance Schedule…………….. (Attachments 5)
-
- Preventive Maintenance Issuance Register…. (Attachments 6)
-
Annexure :
- Annexure-1: Preventive Maintenance Format.
4.0 Responsibilities for Laboratory Instrument:
-
-
The analyst shall be responsible for :
- Inform Head QC for any malfunction/maintenance in the instrument.
-
-
- Make an entry in instrument usage logbook and in “Instrument History Card”.
-
- Prepare the Preventive maintenance schedule and carry out Preventive maintenance as per schedule.
-
- Make an entry in “Preventive Maintenance History Card”.
-
- Carry out calibration whenever required.
-
Quality Control Head or Designee shall be responsible for :
- Inform Maintenance Dept. or instrument manufacturer for the maintenance.
-
- Ensure the proper documentation of maintenance as per SOP.
-
- Ensure the Preventive maintenance done as per the schedule and as per SOP.
-
Quality Assurance person shall be responsible for :
- To ensure that the implementation of the system as per SOP.
-
Regulatory Affairs Quality Head and Plant Head or Designee shall be responsible for:
- To review and approve the SOP.
Also read: SOP for Audit Trail Review and Privilege Policy
5.0 Procedure for Laboratory Instrument:
-
-
Maintenance Procedure :
- If the malfunction or any problem found in any of the laboratory instrument analysts shall inform to Head QC or Designee for the same.
-
-
- The analyst shall keep the label of “UNDER MAINTENANCE NOT TO BE USED” (Attachment-1) with sign and date.
-
- Head QC shall instruct the analyst to make an entry in the “Instrument/Equipment usage log” for “Under Maintenance” in the “Remark” column.
-
- Entries shall be legible and in clear handwriting.
-
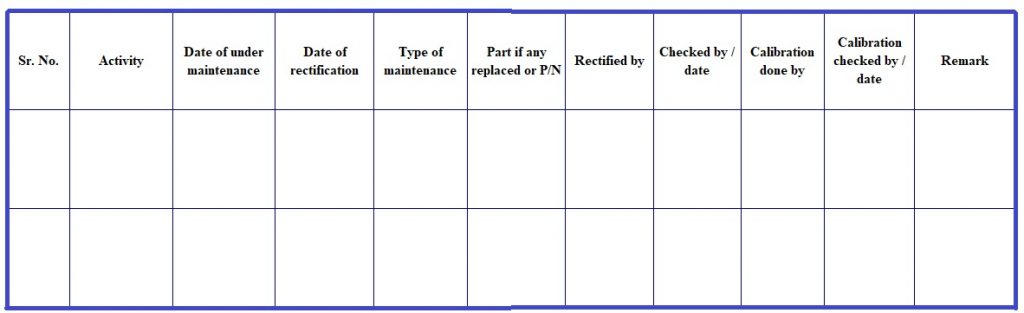
- The analyst shall also make an entry in “INSTRUMENT HISTORY CARD” (Attachment-4) for “Sr.No.”, “Date of under maintenance”, “Type of maintenance”.
-
- The analyst shall fill the details in the Maintenance memo in duplicate and hand over to Head QC or Designee.
-
- For details of the Maintenance memo, refer to the SOP of the Breakdown Maintenance procedure.
-
- Head QC or Designee shall verify the filled matter and put initial/date/time and send it to the Engineering Dept.
-
- Head Engineering or Designee shall put initial/date/time in the “Received By” column and keep one copy with him and Return remaining to QC Dept.
-
-
If required, Head QC or Designee informs to service engineer (Manufacturer) for the rectification.
-
-
- Service engineer (Manufacturer) shall issue a service report duly sign after rectification.
-
- After rectification of instrument, Head QC or Designee shall ensure the working of the instrument and instruct analyst to check the calibration (If applicable) as per the specified procedure.
-
- After the satisfactory calibration of the laboratory instrument, the analyst shall submit the data for checking and for approval.
-
- Analyst shall make entry in a “ INSTRUMENT HISTORY CARD” for “Part if any replaced or P/N”, “Rectified by/Date” (Initial or name of manufacturer, who rectified the instrument”), “Date of Rectification”, “Calibrated by/Date”, “Checked by/Date” and“Remark”.
-
- Head QC or Designee shall instruct the analyst to remove the “UNDER MAINTENANCE, NOT TO BE USED” label and usage of the instrument can be started.
-
Preventive Maintenance Practices for Laboratory Instrument:
- Head QC or Designee shall prepare the Preventive Maintenance format as per respective SOP/instrument instruction manual or discussion with service engineer for instruments.
-
- Preventive Maintenance format as per Annexure-1shall contain columns of “Prepared by”, “Checked by”, “Approved by ”, “Reference SOP No.”, “Type of Instrument” , “Model”, “Make”, “Instrument Code no.”, “Date of PM”, “Next Due date of PM”, “Carried out By/Date” and “Verified By/Date”.
-
- The designee shall issue the Preventive Maintenance format when laboratory instrument Preventive Maintenance is required and put the stamp of “Issuance No.”, “Issued By” and “date”.
-
- Preventive Maintenance Issuance Register shall contain columns of “Issuance No.”, “Issued to”, “Name of PM format”, “Instrument Code No.”, “Issued on”, and “issued by”. (Attachment-6).
-
- Issuance No. shall be allotted as “XXXX/YY”.
-
-
- Where,
-
-
-
- “XXXX” stands for serial no. of format issued in the respective year.
-
-
-
- “YY” stands for the year.
-
-
-
- The designee shall follow the procedure of respective Preventive maintenance form of laboratory instrument for the preventive Maintenance.
-
-
-
- After completion of PM fill the Preventive Maintenance format and submit for verification to the Designee.
-
-
-
Preventive Maintenance Procedure for Laboratory Instrument:
- The analyst shall prepare the preventive maintenance schedule for the coming year at the end of the current year for the major and critical instruments in consultation with Head Quality control.
-
-
- The designee shall update the preventive maintenance schedule for the new laboratory instruments after completion of the performance qualification.
-
-
The frequency of preventive maintenance for a laboratory instrument shall be every 6-month.
-
-
- Preventive maintenance of laboratory instrument shall carry out within ±1 month of the scheduled date.
-
- After completion of preventive maintenance, the analyst shall put initial/date and verified by Head QC or Designee in the schedule.
-
- On the scheduled date, the analyst shall put the label as ‘UNDER PREVENTIVE MAINTENANCE’ (Attachment-2) on the respective instrument.
-
- Analyst/Executive shall call Service engineer for preventive maintenance or Analyst shall carry out Preventive maintenance as per the work description.
-
- The analyst shall procure required spare parts (If required to change) in advance for the preventive maintenance.
-
- The analyst shall make an entry in “PREVENTIVE MAINTENANCE HISTORY CARD” for “Sr. No.”, “Work Description”, “Actual Work Carried Out” (Shall make “Initial/date” in 6 months or in the 12-month column if work carries out satisfactory),” Due Date”, “Date of Preventive Maintenance Carry Out”, “Done By /Date”. If found any abnormalities then make an entry in the “Remark” column and inform to Head QC.
-
- The analyst shall give the “PREVENTIVE MAINTENANCE HISTORY CARD” to Head QC for the checking.
-
- The analyst shall calibrate the laboratory instrument if required and make the necessary entry in respective calibration log.
-
- After preventive maintenance of the laboratory instrument, Head QC or Designee shall ensure the working of the instrument.
-
- Head QC or Designee shall put the initial and date in “PREVENTIVE MAINTENANCE HISTORY CARD” in the “Checked By/Date” column and instruct analyst to remove the “UNDER PREVENTIVE MAINTENANCE” label and usage of the laboratory instrument can be started.
-
Relocation of Laboratory Instrument :
- In the case of instrument relocation, fill the “INSTRUMENT HISTORY CARD”.
Attachments 1 – Under Maintenance Label

Attachment 2 -Under Preventive Maintenance Label

Attachments 3 – Preventive Maintenance History card
Name of Instrument : ___________ Make : ___________
Code No. Of Instrument : ___________ Model : ___________
| Sr. No. | Work Description | Actual Work Carried Out | Due Date | Date Of Preventive maintenance Carried out | Done By/Date | Checked By/ Date | Remarks | ||||
| 6M | 12 M | 6M | 12 M | 6M | 12 M | 6M | 12 M | ||||
Attachments 4 – Laboratory Instrument History card
| Instrument :______________________________ | Code No. :______________________________ |
| Make :__________________________________ | Model : _______________________________ |
| Installation on : ____________________________ | Page No. :_____________________________ |
| Name of service agency : _____________________________________________________________ | |
Attachments 5 – Preventive Maintenance Schedule
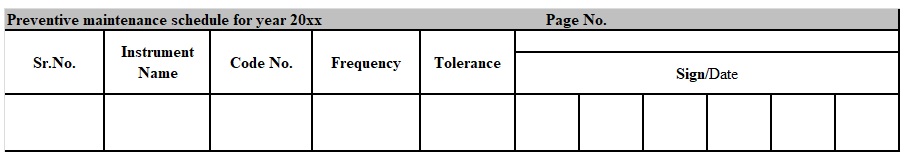
XX : To be mentioned last two digits of the year.
History
| Sr. No. | Date | History details | Name of Instrument | Code No. | Done by/Date | Approved by/date |
Prepared by/Date: _________ Checked by/Date: _________ Approved by/Date:___________
Attachments 6 : Preventive Maintenance Issuance Register
| Issuance No. | Issued to | Name of PM format | Instrument Code. No. | Issued on | Issued By |
Next to read :
- Receipt and Storage of Raw Material
-
- Receipt and Storage of Raw Material
-
- HPLC Calibration- A Complete Guide.
-
- Calibration of UV Spectrophotometer
-
- Procedure for Laboratory Glassware Cleaning.
-
- Quality Control Samples.
-
- Good Laboratory Practices for Workbench
-
- Functions and Reporting system of Quality Control Department in Pharmaceuticals.
-
- Raman Analyzer – Handling Procedure (SOP)



Pingback: Particle Size Analyzer (Malvern) Operation Calibration - Pharma Beginners
Pingback: SOP for Incident / Deviation Management - Pharma Beginners
Pingback: Waters HPLC System-Operation SOP with Empower - Pharma Beginners
Pingback: Micro Balance - Operation and Calibration SOP - Pharma Beginners
Pingback: Operation and Calibration of pH Meter - Pharma Beginners
Pingback: Auto Titrator - Operation and Calibration SOP - Pharma Beginners
Pingback: LOD Oven - Operation and Maintenance SOP - Pharma Beginners
Pingback: SOP for Instrument Calibration (Internal & Third Party) - Pharma Beginners
Pingback: SOP for Audit Trail Review and Privilege Policy - Pharma Beginners