 Standard Operating Procedure (SOP) for Analytical Solution Stability. Procedure for stability study of solutions such as standard preparation, impurity solution, and system suitability solution, etc.
Standard Operating Procedure (SOP) for Analytical Solution Stability. Procedure for stability study of solutions such as standard preparation, impurity solution, and system suitability solution, etc.
SOP for Analytical Solution Stability
1.0 PURPOSE:
-
- The purpose of this SOP is to define the procedure for the solution stability (Use before date determination of solution(s) used in the quality control laboratory.
2.0 SCOPE:
-
- This procedure is applicable to all types of volumetric solution, buffer solution, indicator solution, reagent solution, colorimetric solution, test solution (standard solution for the limit test), mobile phase & diluent.
3.0 REFERENCES:
-
- SOP for Protocol & Report Numbering and Issuance System
-
- SOP for Preparation and handling of Buffers, Indicator, Colorimetric solution, Limit standard solution, Volumetric solutions, and reagents.
4.0 RESPONSIBILITY:
-
-
The analyst shall be responsible for:
- Prepare the analytical solution as per the procedure defined in the respective template and pharmacopeia.
-
-
- Fill the relevant details related to analytical solution preparation in the respective template and logbook.
-
- Affix the label on the bottle “SOLUTION FOR STABILITY STUDY” with the name of the analytical solution.
-
- Examine the solution for any odd observation such as the solution has turned hazy, cloudy with extraneous matter, layer separation.
-
- Report the Head QC or designee about the observation for the further
-
Head QC or Designee shall be responsible to ensure the:
- Availability of all solution preparation and stability study procedure.
-
- Proper practices are followed as per the SOP.
-
- Solutions are prepared and monitored as per the designed protocol.
-
- Proper labeling on the solution/reagent bottle.
-
- All solution preparation records are properly maintained.
-
Quality Assurance shall be responsible for:
- Ensure the implementation of the system as per SOP.
-
- Review and approve SOP.
-
Site Quality Head or Designee shall be responsible for:
- Ensure the implementation of the system as per SOP.
4.0 ABBREVIATIONS USED IN SOP FOR SOLUTION STABILITY:
-
- NA: Not applicable
-
- No.: Number
-
- QA: Quality Assurance
-
- QC: Quality Control
-
- Res: Respective
-
- SOP: Standard operating procedure
5.0 PROCEDURE FOR SOLUTION STABILITY:
-
- The analyst shall assign the protocol number as per respective SOP for Protocol & Report Numbering and Issuance System.
-
- Prepare the protocol for the respective analytical solution as per Annexures-1.
-
- Prepare and standardize the
-
-
- Volumetric solution,
-
-
-
- Buffer solution,
-
-
-
- Indicator solution,
-
-
-
- Reagent solution,
-
-
-
- The colorimetric solution as per SOP
-
-
- Preparation and handling of buffers, indicator, colorimetric solution, limit standard solution, volumetric solution, and reagents.
-
- Fill all the relevant details related to analytical solution preparation in the respective template/logbooks.
-
- Affix the label on the bottle “SOLUTION FOR STABILITY STUDY” with the name of the analytical solution.
-
- Carry out the stability study of analytical solution(s) as per Annexure-3 but not limited to.
-
- Carry out the stability study through an approved stability protocol.
-
- After the preparation of the analytical solution, the analyst shall examine the solution for any odd observation such as the solution has turned hazy, cloudy, with extraneous matter, layer separation, etc.
-
- Record the observation in the stability study Annexures-2 for the respective analytical solution and submit the same to the section head/reviewer.
-
- In case the solution has turned hazy, cloudy, with extraneous matters, layer separation, or any other odd observation, the analyst shall immediately report to Head QC or designee and Head QC or designee shall decide further action.
-
- The analyst shall perform stability of solution as per the procedure define in the respective protocol and record the observation/results.
-
- Evaluate the observation/results filled in protocol against the acceptance criteria defined in the stability study protocol.
-
- Define the use before the date of the solution based on the solution stability study data.
-
- After completion of the stability, the study record shall be maintained in the register as per Annexure- 4.
-
- Stability study of mobile phase & diluent shall be performed at the time of method validation.
6.0 ANNEXURES:
-
- Protocol for stability study of analytical solutions(Annexure 1).
-
- Observation table for the analytical solution(Annexure 2).
-
- List of Analytical Solutions(Annexure 3).
-
- Format for Stability study register(Annexure 4).
Protocol for stability study of analytical solutions(Annexure 1)
Page: 1
| Title : | Page No. |
| Protocol No.: | Effective Date: |
Stability study
Protocol for Analytical Solution
Name of Analytical Solution:_________________________________________
Page 2 :
INDEX
| Sr. No. | Description | Page No. |
| 01 | Cover Page. | |
| 02 | Index | |
| 03 | Protocol Approval | |
| 04 | Overview. | |
| 05 | Procedure | |
| 06 |
Acceptance Criteria |
|
| 07 | Assignment of shelf life | |
| 08 | Failure to meet the acceptance criteria | |
| 09 | Evaluation of report | |
| 10 | Conclusion | |
| 11 | Summary report |
Page 3
PROTOCOL APPROVAL
|
NAME |
SIGNATURE /DATE |
|
| Prepared By: Officer, Quality Control | ||
| Reviewed By: Executive, Quality Control | ||
| Reviewed By: Head, Quality Control | ||
| Approved By: Head, Quality |
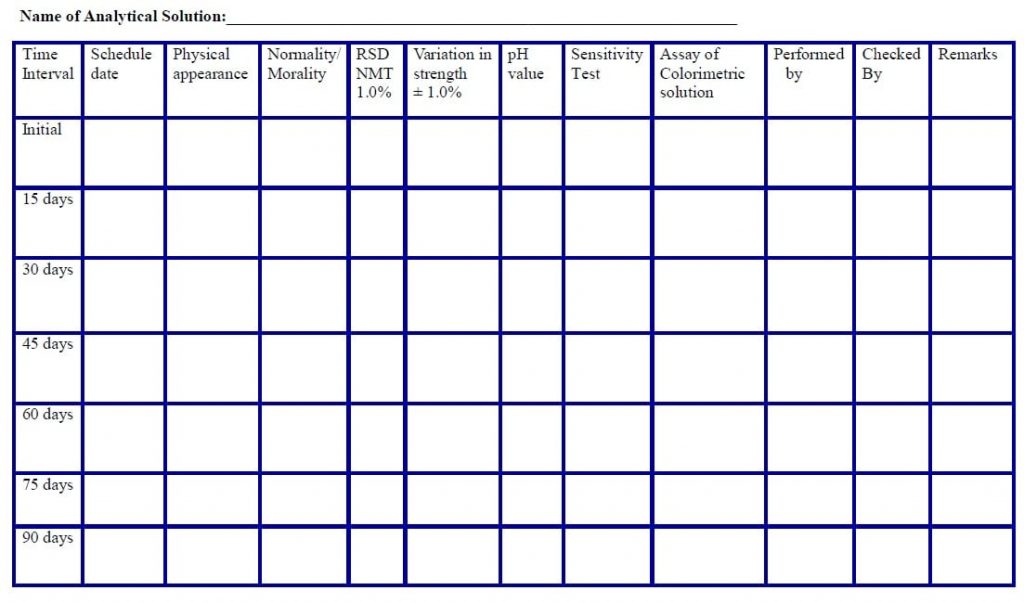
Observation table for the analytical solution (Annexure 2)
List of Analytical Solutions(Annexure 3)
| Sr.No. | NAME OF VOLUMETRIC SOLUTIONS |
| 1 | AMMONIUM THIOCYNATE 0.1M |
| 2 | CERIC AMMONIUM NITRATE 0.1M |
| 3 | CERIC AMMONIUM SULPHATE 0.1M |
| 4 | FERROUS AMMONIUM SULPHATE 0.1M |
| 5 | IODINE 0.05M |
| 6 | LEAD NITRATE 0.1M |
| 7 | PERCHLORIC ACID 0.1M |
| 8 | HYDROCHLORIC ACID 1.0M |
| 9 | HYDROCHLORIC ACID 0.5M |
| 10 | HYDROCHLORIC ACID 0.1M |
| 11 | SILVER NITRATE 0.1M |
| 12 | SODIUM HYDROXIDE 1.0M |
| 13 | SODIUM HYDROXIDE 0.1M |
| 14 | DISODIUM EDETATE 0.05M |
| 15 | TETRABUTYL AMMONIUM HYDROXIDE 0.1M |
| 16 | SODIUM THIOSULPHATE 0.1 M |
| 17 | SULPHURIC ACID 0.05M |
| 18 | DISODIUM EDETATE 0.1M |
| 19 | ZINC SULPHATE 0.1M |
| Sr.No. | NAME OF GENERAL REAGENTS |
| 1 | AMMONIA SOLUTION DILUTE |
| 2 | CHLORIDE SOLUTION STANDARD (0.0824% W/V NaCl, 500 PPM) |
| 3 | AMMONIA -AMMONIUM CHLORIDE BUFFER |
| 4 | SULPHATE STANDARD SOLUTION 10 PPM ETHANOLIC |
| 5 | DIPHENYLAMINE SOLUTION |
| 6 | DISODIUM EDETATE 0.01 M |
| 7 | AMMONIA SOLUTION 6 M |
| 8 | NITRIC ACID 2 M |
| 9 | AMMONIA SOLUTION 10 M |
| 10 | DILUTE AMMONIUM CHLORIDE SOLUTION (NESSLER’S) |
| 11 | POTASSIUM PERMANGANATE 0.02 M |
| 12 | FERRIC CHLORIDE COLORIMETRIC SOLUTION (FCS) |
| 13 | NINHYDRIN SOLUTION |
| 14 | ACETIC ACID-AMMONIUM ACETATE BUFFER |
| 15 | ARSENIC STOCK 1000PPM |
| 16 | EOSIN SOLUTION |
| 17 | SULPHURIC ACID 1 M |
| 18 | CUPRIC SULPHATE COLORIMETRIC SOLUTION (CSS) |
| 19 | LEAD STANDARD SOLUTION 0.1% Pb (1000 PPM) |
| 20 | IODINE SOLUTION 0.1 M |
| 21 | HYDROCHLORIC ACID AsT BROMINATED |
| 22 | ACETATE BUFFER PH 3.5 |
| 23 | POTASSIUM MERCURI IODIDE SOLUTION ALKALINE |
| 24 | AMMONIA BUFFER PH 10.0 |
| 25 | THIOCETAMIDE SOLUTION |
| Sr.No. | NAME OF GENERAL REAGENTS |
| 26 | MERCURIC ACETATE SOLUTION |
| 27 | POTASSIUM DICHROMATE SOLUTION DILUTE |
| 28 | 1- NAPHTHOLBENZEIN SOLUTION |
| 29 | AMMONIUM STANDARD SOLUTION 100 PPM |
| 30 | STANDARD SUSPENSION STOCK |
| 31 | IRON STOCK 1000 PPM |
| 32 | POTASSIUM CHLORIDE 10% W/V |
| 33 | BARIUM CHLORIDE SOLUTION 10% W/V |
| 34 | POTASSIUM IODIDE SOLUTION DILUTE 10 % W/V |
| 35 | HYDROCHLORIC ACID 0.01M |
| 36 | NITRATE STANDARD SOLUTION 1000PPM |
| 37 | SULPHATE STANDARD SOLUTION 1000 PPM |
| 38 | COBALTOUS CHLORIDE COLORIMETRIC SOLUTION (CCS) |
| 39 | HYDROCHLORIC ACID 2.0 M |
| 40 | DILUTE NITRIC ACID |
Format for Stability study register(Annexure 4)
|
Sr.No |
Date | Name of
Analytical Solution |
Protocol
No |
Stability
Period |
Stability Study Performed By |
Checked By |



Pingback: SOP for Working/Reference Standard Qualification - Pharma Beginners
Pingback: Operation and Calibration of pH Meter - Pharma Beginners
Pingback: SOP for Audit Trail Review and Privilege Policy - Pharma Beginners