Standard Operating procedure (SOP) for rounding off figures, numbers of analytical test results, including assay result rounding off, related substances (RS) result and other laboratory generated quantitative value and their reporting system.
SOP for Rounding Off Test Results
1.0 PURPOSE:
-
- The purpose of this SOP is to describe the standard for “Rounding off” and reporting of numerical values related to analysis and batch records.
2.0 SCOPE:
-
- This SOP is applicable for Rounding off and Reporting of Numerical values during any testing carried out in Quality Control department or any calculation in the other department of manufacturing unit.
3.0 REFERENCE:
-
- TGA Guideline on Rounding of figures (Guideline 18).
- FDA – ORA Laboratory Volume III, Section 4, Title : Basic Statistics and Data Presentation.
4.0 RESPONSIBILITY:
-
- Analyst shall responsible for:
- Performing calculations and report results in accordance with this guidance.
- Analyst shall responsible for:
-
- Quality Control Head or Designee shall responsible for:
- Ensuring that all personnel who perform analysis and record data are trained on “rounding off” and reporting procedures to determine compliance of the results/conformity of the batch under analysis.
- Quality Control Head or Designee shall responsible for:
-
-
- Ensuring that all personnel performing the calculations and reporting results follow this SOP.
-
-
- QA Head or Designee shall be responsible for:
- Overall implementation of this SOP at the site.
- QA Head or Designee shall be responsible for:
5.0 ABBREVIATIONS:
-
- LC-MS : Liquid Chromatography- Mass Spectrometry.
-
- MS : Mass Spectrometry.
-
- SOP : Standard Operating Procedure.
-
- STP : Standard Testing procedure.
6.0 DEFINITION:
-
- Calculated Value:
- An un-rounded number that is the result of a calculation.
- Calculated Value:
-
- Reportable Value:
- The final result derived from one full execution of an appropriately defined, written, approved test method /manufacturing procedure after rounding off the calculated numerical value as per procedure .
- Reportable Value:
-
-
- The reportable value is the end result of a completed measurement procedure, as documented.
-
-
- Significant Figure:
- Significant Figures are the digits in a numerical value that should consider when rounding a result.
- Significant Figure:
Also read: SOP for Audit Trail Review and Privilege Policy
7.0 PROCEDURE:
-
- Numerical values observed/calculated during analysis/manufacturing are compared with stated limits to determine whether there is conformance with compendial or in-house test specification/acceptance criteria requirements.
-
- The observed or calculated values usually contain more figures than in the stated limit and a reportable result is to be rounded off to the number of significant figures that is in agreement with the limit expression.
-
- Based on the significance of the numerical value, a number of conventions need to be taken into consideration while reporting / “rounding off” of the same.
-
- Get the details the following paragraphs.
-
Significant Figures:
- The number of significant figures in a result implies a given degree of confidence in that result.
-
-
- Generally, the number of significant figures reported should relate to the precision expected for the measurement.
-
-
-
- The number reported should be at least as precise as the measurement.
-
-
-
- Limits expressed in monograph definitions and tests, regardless of whether the values are expressed as percentages or as absolute numbers, are considered significant to the last digit shown.
-
-
-
- Monographs, Standard Operating Procedures (SOPs), Standard Testing Procedures (STPs) or other applicable procedures normally specify how a result should be expressed.
-
-
-
- Where no formal guidance is given (in the Monograph/SOP/STP), the following criteria must be used to determine the significant figures in a numerical value which need to be considered while rounding off:
-
-
-
-
Any non-zero number shall consider significant.
-
-
For example: in 354 = 3, 5 and 4 are significant figures
-
-
-
A zero surrounded by non-zero numbers shall consider significant.
-
-
For example: In 1001 = 1, 0, 0 and 1 significant figures
-
-
- A zero directly to the left of a non-zero number shall not considered significant, except when surrounded by non-zero numbers or decimals.
-
For example:
1) In 0.961 = 9, 6 and 1 are significant figures
2) In 0.0961 = 0 (to the right of the decimal point), 9, 6 and 1 are significant figures
3) In 4.0961 = 4, 0, 9, 6 and 1 are significant figures
-
-
- Zero values to the right of a non-zero number shall consider significant if the limit expression extends to the same amount of decimal places.
-
For example:
1) 35.0 = 3, 5 and 0 are significant figures
2) 35.00 = 3, 5, 0 and 0 are significant figures
-
-
General Rules for “Rounding Off” and Reporting of Numerical Values:
-
Note: General rules to be followed during “Rounding Off” and reporting of numerical values are as follows (exceptions to these rules are those that are specified otherwise in the Monograph, SOP or STP).
-
-
- Remember limits are fixed numbers. and hence, these shall not “rounded off”.
-
-
-
- Do not rounding off until the completion of final calculations for reportable value.
-
-
-
- Intermediate calculations (e.g. slope for linearity) may rounded for reporting purposes,
-
-
-
- But use the original value (un-rounded) for any further calculations, unless otherwise specified.
-
-
-
For example, when Assay needs to report as mg/tab and % label claim, then the rounding off the figures as follows:
-
-
-
- In the event Assay is to be reported in (mg/tab): If the assay value arrived at is 25.467 mg/tab.
-
-
-
- Then, the value to be reported for mg/tab after rounding off is: 25 mg/tab.
-
-
-
- In the event Assay is to be reported in % label claim i.e. Assay (% label claim) = (Assay in mg/tab)*100/Claim.
-
-
-
- In this case, the value to be taken for Assay in mg/tab Should be 25.467 and not 25 (i.e. after rounding).
-
-
-
- That is, the formula becomes: Assay (% label claim) = (25.467*100/Claim) and not (25*100/Claim)
-
-
-
- However, in case the intermediate value has more than 4 decimal places, then the intermediate value shall be used, “as such”, i.e. it is recommended to consider 4 decimal places,
-
-
-
- where the results with truncated values do not alter the final results when calculated with non-truncated value, e.g. if Loss on Drying (LOD) value is 1.2345678912, then in calculation, Use the following value : 1.2345.
-
-
-
-
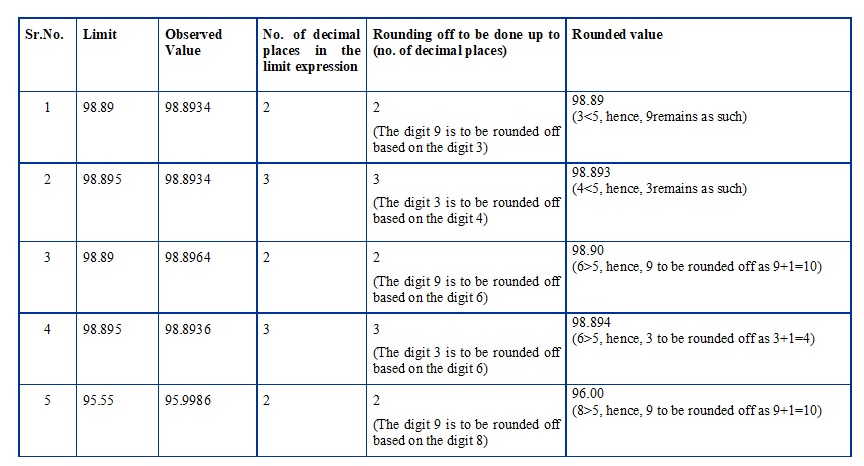
The observed or calculated values / test results shall “rounded off” to the number of places in agreement with the limit expression by the following procedure:
- When “rounding off” of results is required, check the digit in the decimal place to the right of the last place in the limit expression.
-
-
-
- If this digit is smaller than 5, it must be eliminated and the preceding digit shall remain unchanged. (No rounding off is to be done).
-
-
-
- If this digit is 5 or greater than 5, it must be eliminated and the preceding digit shall be increased by one (Rounding off to be done).
-
Some examples of implementation of the above said principles while rounding off, are given in the following table:
-
- Example : 11.5 + 11.65 + 9.90 = 33.05
- If the limit expression is without a decimal place, the data shall rounded off and reported as a whole number,
- Example : 11.5 + 11.65 + 9.90 = 33.05
-
-
- e.g. Limit for a particular parameter is: Not More Than (NMT) 400, Report the result as: 243.
-
Sums and Differences:
- When adding or subtracting, the number of decimal places in the result shall be the same as the number of decimal places in the component with the fewest decimal places.
-
-
-
- Since the number of decimal places in the component with the fewest decimal places (here 11.5) is 1, the no. of decimal places in the result should also be rounded off to 1.
- Hence, the result shall be reported as 33.1.
- Since the number of decimal places in the component with the fewest decimal places (here 11.5) is 1, the no. of decimal places in the result should also be rounded off to 1.
-
-
-
Products and Quotients:
- When multiplying or dividing, the result shall have no more significant figures than the measurement with the smallest number of significant figures entering into the calculation.
-
-
-
- Example: 4.266 x 21 = 89.586
-
Since the number of significant figures in the component with the fewer number of significant figures (here 21) is 2, the result also shall be rounded off to 2 significant figures. Hence, the result shall be reported as 90.
Note: In both cases above (section 7.5.4.5 and 7.5.4.6), the number of decimal places in the final reported results must however be in agreement with the limit expression.
-
- In case a result (numerical value) is obtained through an instrument output, the same result (numerical value in this case) should be reported with the exact number of decimal places as shown by the Instrument in the raw data sheet/laboratory notebook.
-
- However, the rounded value needs to be shown in the Certificate of Analysis (CoA) / Final Reporting Sheet.
-
“Rounding Off” and Reporting of Numerical Values during conversions:
- Conversion of Weight (kg /gm etc.) into Units (numbers):
-
-
- If weight is to be converted into number of units and the calculated number of units is obtained in decimal values,
- then, “rounding off” must be done by converting it to a whole number without changing the last digit before decimal, irrespective of the digit after decimal (i.e. whether the digit is greater than or equal to or less than 5).
-
Examples are shown below:
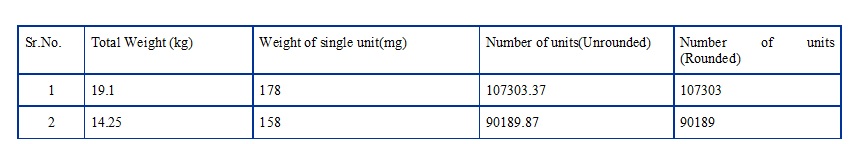
Note: These clauses however do not apply to average weights.Conversion of Number of Units into Weight (kg/gm etc.):
-
-
- Weighing balances used for recording weights must be suitable to display the number of decimal places as intended for the purpose of its use based on the operation range.
-
-
-
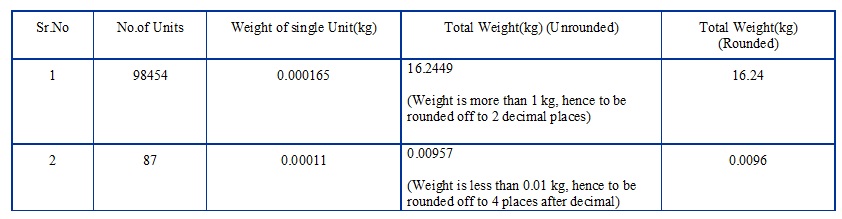
- General rules for reporting/rounding off of weights after conversion from number of units; unless otherwise specified.
-
-
-
- Weights Less Than or equal to 0.01 kg: Minimum 4 places after decimal.
-
-
-
- If weights More Than 0.01 kg but less than 1 kg: Minimum 3 places after decimal.
-
-
-
- Weight More Than or equal to 1 kg: Minimum 2 places after decimal.
-
-
-
Do the rounding off as per the following convention:
-
-
-
- If the digit in the decimal place to be rounded off is <5, then the value (of the weight calculated) shall be rounded off without changing the digit preceding the digit to be rounded off.
-
-
-
- If the digit in the decimal place to be rounded off is ≥ 5, then the value (of the weight calculated) shall be rounded off by adding 1 to the digit preceding the digit to be rounded off.
-
For Example:
-
-
“Rounding Off” and Reporting of Numerical Values in Manufacturing processes:
- Record all batch records entries to the same significant digits as the limit / ranges in the batch record.
- When a weight slip is required, the batch record must contain the value as shown in the print out along with the rounded value.
- When performing Active Pharmaceutical Ingredient (API) calculations for ispensing purposes, if the quantity specified has 3 places after decimal, calculations and dispensing activity must take into account the 3 decimal places. However, as a general rule (unless otherwise specified), weight must be reported/rounded off.
-
Rounding may be done as follows:
- When “Rounding Off” of results is required, check the digit in the decimal place to the right of the last place in the limit expression.
- If the digit is smaller than 5, it must be eliminated and the preceding digit shall remain unchanged. (No rounding off is to be done).
- If the digit is 5 or greater than 5, it must be eliminated and the preceding digit shall be increased by one. (Rounding off to be done).
- Illustrations of Rounding Off of Numerical Values for Production are given in the table below:
-
| Batch Record Require | Un rounded value | Rounded Results | Conformity of Rounding Off (With Respect to number of
decimal places reported and rounded off the digit)
(Yes/No) |
| Yield limit
95 – 101% |
98.59%
|
99%
|
Yes |
| 98.59% | 98.60% |
No (Since limit is without any decimal place) |
|
| Bulk Solution
800 liters |
788.6 liters | 788.6 liters |
No (Since limit has no decimal places,rounded value also must have no decimal place) |
|
788.6 liters
|
789 liters
|
Yes | |
| Weight of Filled Bottles
823.74 – 878.40 |
853.505 | 854.00 | No
(The digit corresponding to the last decimal place in the limit. Rounding off the value based on the digit to its right) |
|
853.505
|
853.51
|
Yes
|
|
| Theoretical weight of API 33.000 kg | 33.09920 kg | 33.0992 kg |
No (The limit has 3 decimal places, hence the rounding of value up to 3 decimal places) |
|
33.09559 kg
|
33.096 kg
|
Yes
|
-
“Rounding Off” and Reporting of Analytical Values:
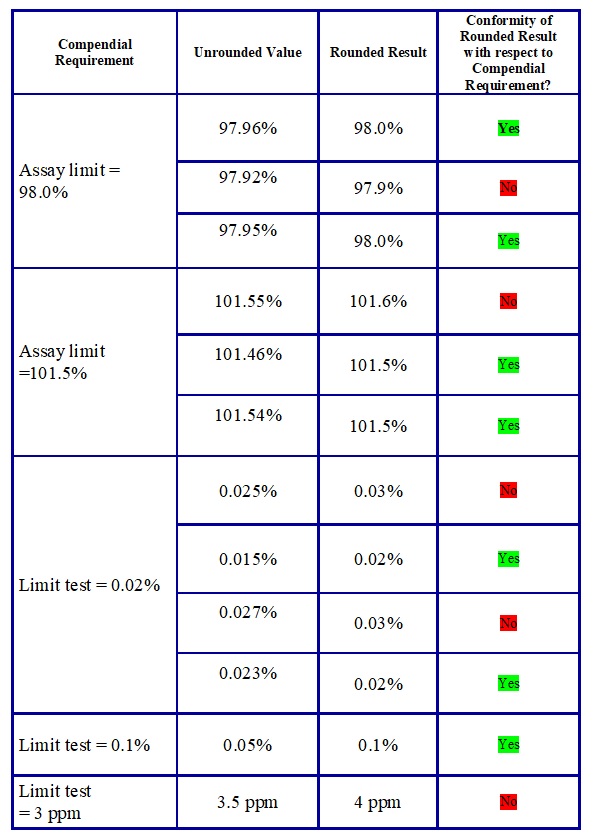
- A table showing illustrations of “rounding off” of analytical values for comparison with limits. (Refer Annexure 1)
-
- Rounding Off and Reporting methodology for some important analytical parameters. (Refer Annexure 2).
-
- If the specification gives more than one unit of reporting (e.g. mg and %), results should be reported in all the units given.
-
- Specification Setting:
- During setting or revision of specifications, in case rounding off needs to be done, the most conservative approach shall follow by rounding off the lower specification limit and the upper specification limit towards the Median of the range or Target Value .
- Specification Setting:
For Example:
-
-
- Supposing specification limits are 20.163 mg to 22.167 mg.
-
-
-
- If there is a need to round off these values to 2 digits after decimal,
-
-
-
- then the rounded values will be: 20.17 to 22.16 (and not 20.16 mg to 22.17 mg as per the general rules of rounding off).
-
-
-
- This is because if the general rounding off rule is followed, the specification limits will move outside the acceptance limit.
-
-
- Illustrations of rounding of numerical values while reporting results of each parameter during summary preparation of method validation studies in Annexure 3.
-
- The number of decimal places in the specification limits shall set in line with this SOP to help the analyst information to reporting of the number of decimal places.
-
- In cases where there are no defined specifications available for analytical parameters, general acceptance criteria for the same shall be considered while reporting decimal places.
-
- Rounding off of parameters with very low numerical values
- For example: concentration of Genotoxic impurities monitored by LCMS/ MS).
- Rounding off of parameters with very low numerical values
-
-
- If on rounding off such parameters, the value becomes zero or same for two or more results (resulting in inconclusive comparison of results),
-
-
-
- Then do the rounding off in such a way that at least two numeric values are retained after preceding zeroes.
-
-
-
- Illustrated in the examples shown below:
-
Case-I
On rounding up to three decimal places, the values becomes zero
| in μg per g | Rounding up to three places | Rounded Value |
| Concentration of LOD –level -10.0000912 | 0.000 | 0.000091 |
| Concentration of LOD -level -20.0000433 | 0.000 | 0.000043 |
Case-II
| in μg per g | Rounding up to three places | Rounded Value |
| Concentration of LOD –level -10.000912 | 0.001 | 0.00091 |
| Concentration of LOD -level -20.000733 | 0.001 | 0.00073 |
-
- In case of customer specific requirement or for trending purposes, if the number of significant digits in a numerical value is to be increased than what is specified in this SOP, it may do so after approval for the same from the QA Head.
8.0 ANNEXURE:
Annexure – 1
Illustrations for Rounding Off of Numerical Values for Comparison with Requirements
Annexure – 2
Rounding Off and Reporting Methodology for some important Analytical Parameters
-
-
General (applicable for all tests)
-
| Reporting of Results | Example | |
| Limit | Rounded Value of Result | |
| The limits in the specification should always be taken into account while reporting any values on the Certificate Of Analysis (CoA) or stability data sheets.
Whenever rounding off is done on the lower side, USP General Notices, European Pharmacopoeia (Ph. Eur), British Pharmacopoeia (BP) to be referred to ensure that rounding off is not misleading to change a failing result to a passing result. |
_ | _ |
-
-
Assay Test
-
| For API: Reporting must be in percentage, unless otherwise specified. The number of decimal places must be in line with the limits, which is generally only 1.
For Drug Products: Assay values of API, active and inactive excipients must be reported in mg as well as in percentage. 237.6 – 262.6 mg 95.0 – 105.0% 248.6 mg 99.4% Reporting in mg (or other similar units) must be up to the decimal place which takes care of the % value in the specification. Accordingly, the proposed number of digits after decimal to be reported for various strengths of drug product are tabulated below (E.g. given in mg.): <1mg : 4 digits > 1mg and < 10 mg : 3 digits > 10 mg and < 100 mg: 2 digits > 100 mg: 1 digit If the results are to be reported in both, mg & % (for example), the un-rounded recorded value will be taken into account to calculate both the above values for reporting. Assay value in percentage must be reported with only one digit after decimal, unless otherwise specified. |
98.0 to 102.0%
237.6 – 262.6 mg 95.0 – 105.0% 0.4000 to 0.5000 mg 4.200 to 4.800 mg 20.0 0 to 22.00 mg 200.0 to 225.0 mg |
99.1%
248.6 mg 99.4% 0.4854 mg 4.564 mg 21.52 mg 223.5 mg |
-
-
Assay of Preservatives
-
| No. of digits to report after decimal place:
Preservative Level: 0.01% to 0.09 % – Four >0.09% & < 0.9 % – Three >0.9% & <9 % – Two For results to be reported in ppm: · If the result is < 50 ppm, report it with 1 decimal place · If the result is > 50 ppm, report it as a whole no. |
_ | _ |
-
-
Disintegration Time
-
| Reporting should be done in Minutes:
Seconds, as displayed on the instrument |
NMT 10
minutes |
1 minute 15
seconds |
-
- Below Limit of Quantification (BLQ) Results
| Before reporting, check all results against LOQ values given in STP/Validation report, as applicable.
Whenever the results are below LOQ,the numerical value should be reported along with the text: “BLQ or Below LOQ” and additionally, report the LOQ value as a footnote in COA/ data sheet. Additionally, Give a statement that these are to be excluded for reporting / calculations of Total Related Substances. Do not use the symbol of < (less than) . Instead the report same in words as, “less than” or “Below LOQ”. (This shall also be followed in case of values “below disregard limit”. In case value is below disregard limit, then, it shall be clearly stated on CoA that the same is “Below disregard limit”.) |
In case observed value is 0.003% w/w and LOQ is 0.01% w/w.
The result shall be reported as: BLQ* (0.003% w/w) or Below LOQ* (0.003% w/w). *LOQ= 0.01% w/w. |
-
-
Other tests
-
| For the following analytical parameters, the decimal places in the result should be similar to that of the limits outlined in the specifications. | |||
| Bulk
Density/Tapped Density |
Limit is generally up to one decimal place |
Between 2.2 – 2.4 g/ml |
2.3 g/ml |
| Chloride Content | Limit is generally up to one decimal place |
Not less than 10.9% and not more than 11.3% |
11.1% |
| Optical Rotation | Limit is generally up to one decimal place |
Between +15.1° to +17.5° |
+16.8° |
| pH | Limit is generally up to one decimal place |
Between 5.0 to 7.0 |
5.5 |
| Refractive Index | Limit is generally up to three decimal places |
Between 1.520 and 1.524 |
1.522 |
| Specific Gravity | Limit is generally up to three decimal places |
Between 0.996 and 1.002 |
1.000 |
| Average
Weight/Average Fill Weight |
Limit is generally up to one decimal place |
200.5 mg |
200.5 mg |
| Alcohol
Content |
_ |
Between 65% and 75% Between 4.0% and 6.0% |
70%
5.1% |
| Limit Tests | when the same standard is used as the limit.Report as “Complies”
· Provide the numerical values wherever applicable, instead of writing “Complies”. · Symbols like > (more than), < (less than) shall not be used. Instead the same will be reported in words as, “less than” and “more than”. |
NMT 0.002% |
Less than 0.002% |



Pingback: SOP for Handling of Out of Calibration (OOC) - Pharma Beginners
Pingback: SOP for Review of Analytical Report and Raw Data - Pharma Beginners