 Standard Operating Procedure (SOP) for Sampling of Raw Material (API & Excipient) as per Schedule M and ICH Q7 (Good Manufacturing Practices Guideline). Raw Material sampling is the process of abstraction of a representative portion of material or a group of units from a larger quantity of material or collection of units.
Standard Operating Procedure (SOP) for Sampling of Raw Material (API & Excipient) as per Schedule M and ICH Q7 (Good Manufacturing Practices Guideline). Raw Material sampling is the process of abstraction of a representative portion of material or a group of units from a larger quantity of material or collection of units.
Procedure for Raw Material Sampling
1.0 PURPOSE:
-
- The purpose of this Standard Operating Procedure is to describe the sampling procedure of raw materials.
2.0 SCOPE:
-
- This Standard Operating Procedure is applicable to the sampling of all raw materials (solids & liquids) and raw materials due for re-testing at a pharmaceutical manufacturing plant.
3.0 REFERENCES:
-
- Activity, maintenance, and cleaning of the sampling room. (SOP)
-
- Cleaning of sampling devices. (SOP)
-
- Secondary gowning procedure for the primary area. (SOP)
-
- Collection, preservation, and disposal of raw materials/packing materials control sample. (SOP)
-
- ICH Q7A – Guidance of Industry, Good Manufacturing Practices (Guideline)
-
- Near-Infrared Analyzer (NIR). (SOP)
-
- Receipt, testing & release of raw materials and packing materials. (SOP)
-
- Aseptic Technique for Microbiological Testing. (SOP)
-
- SOP for Raman Analyzer.
4.0 RESPONSIBILITY:
-
- Officer or Executive of the QC Department shall be responsible for the preparation of new or revision of existing SOPs.
-
- Head of the department/designee of respective areas & QA shall be responsible for reviewing the SOPs.
-
- Plant Head and Head-Quality shall be responsible for the approval of SOP.
-
- QA shall be responsible for the distribution and control of SOPs to various departments.
5.0 ABBREVIATIONS:
-
- AR. No. : Analytical Report Number
-
- API: Active Pharmaceutical Ingredients
-
- CC: Change Control
-
- GIM: Goods Inward Memo
-
- HPLC: High-Performance Liquid Chromatography
-
- HEPA: High-Efficiency Particulate Air
-
- IR: Infrared
-
- NIR: Near-Infrared
-
- PPE’s: Personnel Protective Equipments
-
- RMS: Raw Material Store
-
- RLAF: Reverse Laminar Air Flow
-
- STP: Standard Testing Procedure
-
- DEFINITION:
-
- Standard Operating Procedure (SOP): A written authorized procedure, which gives instructions for performing operations.
-
- Sampling: Sampling is the process of abstraction of a representative portion of material or a group of units from a larger quantity of material or collection of units.
6.0 PROCEDURE FOR RAW MATERIAL SAMPLING:
-
-
Precautions during raw material sampling:
-
-
- Switch ON the RLAF 15 minutes before sampling.
-
- Keep RLAF “ON” during performing the sampling activity.
-
- Always take one batch (single A.R. No.) at a time into the sampling room.
-
- Switch OFF the RLAF after completion of sampling.
-
- Use PPE’s like gloves and nose masks etc. during sampling of the material.
-
- Perform the Hygroscopic Raw Material in humidity controlled area.
-
- Verify the humidity of the sampling room through a hygrometer available in the area.
-
- In case the humidity level goes out of the limit. Inform to the maintenance for rectification.
-
- Perform the sampling of Hygroscopic raw materials immediately after the opening of the container and close the container immediately after sampling.
-
- Place a small silica gel bag inside the container or the outer side of the double polybag where the hygroscopic material sampled.
-
- Immediately sample the raw material having storage condition between 2°C to 8°C and Place the containers back to the specified storage condition.
-
- Sample the Light-sensitive raw materials under sodium lamp light and collect the sample in a black polybag.
-
- In case where purging of nitrogen is recommended by the vendor then purge the material with nitrogen after sampling and before sealing the bag and container.
-
- QA shall provide the list of light-sensitive materials and materials requiring Nitrogen flushing in the sampling room.
-
- After sampling, the used sampling equipment shall be placed in LDPE polythene bag with label “To be cleaned” to avoid spillage and cross-contamination and shall be taken back to designed area for cleaning by QC Personnel.
-
- Any spillage during the sampling. Clean immediately after sampling.
-
-
General procedure for Raw Material Sampling:
-
-
- Section Head or designee:
-
- On receipt of GIM from the raw material store (RMS), make an entry in raw material register as per the SOP of “Receipt, testing, and release of raw materials and packing materials.
-
- Raise the “consumption order” through Metis/ERP/Other software/Manually in case of any excess sample required for any investigation purpose, preparation of working standard, etc. and take the authorization of Quality head or designee for the re-sampling in Annexure- 4
-
- Allot the work to the analyst for the sampling of raw materials with GIM, sampling report checklist etc.
-
- Identify all the sampling tools i.e., rods and spatulas through number and Maintain its issuance and destruction record as per Annexure-6.
-
- Follow the numbering system as:-
-
-
- For API (sampling rods) : ASR-1, ASR-2, ………..
-
-
-
- API (sampling spatulas) : ASS-1, ASS-2, …………
- For Excipients (sampling rods) : ESR-1, ESR-2, ……….
-
-
-
- Excipients (sampling spatulas) : ESS-1, ESS-2, ………..
-
-
-
- For Liquids ( sampling rods) : LSR-1, LSR-2 ……….
- where,
-
-
- A = Active, SR = Sampling Rod, E = Excipient,SS = Sampling Spatula , L = Liquid
-
- Use an appropriate sampling device for the sampling of material, depending upon the size and length of the containers.
-
- In case the sampling device are not found in a condition appropriate to be used further for sampling (e.g due to rusting, bending, etc.), then it shall be discarded and the destruction records along with the reason for discard and maintain as per Annexure-6.
-
- The number once allocated to a sampling device cannot be reallocated to any other sampling device even after its destruction.
-
-
Analyst:
-
-
- Take the raw material GIM and respective documents folder and proceed for sampling.
-
- Maintain the area status label of the sampling room as per the procedure.
-
- Record the cleaning activity in ‘Raw material sampling and cleaning log’ Annexure-2.
-
- The analyst shall check the status of the sampling room, RLAF , RLAF filter and vacuum cleaner etc.
-
-
Ensure that sampling devices are cleaned & the clean cards are attached as per SOP.
-
-
- Analyst shall check the Quarantine label, manufacturer’s label and COA of the consignment against GIM.
-
- Check the title of material, item code, name of manufacturer and supplier, manufacturer’s lot no./batch no., number of containers, quantity received, manufacturing date, expiry date/ retest date on GIM.
-
- The analyst shall ensure the physical condition of received containers. if any discrepancies found then inform the same to section head or designee.
-
- Sample only after taking clearance/approval from Head QC
-
- The analyst shall check the manufacturer COA before sampling for compliance.
-
- The analyst shall ensure that reading of the HEPA filter pressure is within the limit and make an entry in ‘Raw material sampling and cleaning log’ as per Annexure-2.
-
- Arrange the containers, count the number of containers and also enter the material and sampling detail in the RM sampling report as per Annexure-1.
-
- The analyst shall take the sampling devices, PPE’s (like gloves and nose mask) self-sealing bags or bottles, labels, etc. from the cabinet in the sampling room.
-
- The analyst shall check the integrity of the Silica gel bag where required.
-
- After the verification of all of the above parameters, the analyst shall proceed for sampling.
-
- The sample quantity is defined based on analysis parameters as per STP and the same quantity mentioned as per “Raw material sample quantity justification ” (Annexure-5).
-
- The analyst shall keep the analytical sample 1.2 times and control sample quantity 2.1 times the quantity of sample required to conduct all tests as per the specification.
-
- Do not keep the control sample of solvents and retest materials.
-
-
The Sampling of Raw Material:
-
-
- For Excipient – Take the sample according to √n+1, Where ‘n’ is the no. of received containers per A.R. No.
-
- For API received from other than the same group of companies (Any other location) perform the identification test by NIR/Raman Analyzer of all containers and collect the sample for analysis and control sample according to √n+1.
-
- If the identification test is not performed by NIR/Raman Analyzer instrument during the sampling then take a representative sample from each container in a separate polybag (approximately 5 mg) which is sufficient to perform IR and write the A.R.No. and material details on polybag with marker except for the solvents
-
- The identification test by NIR/Raman Analyzer can be performed during the receipt of consignment or during the sampling of materials.
-
- For API received from an approved vendor (As per SOP of Vendor Management), if identification by NIR/Raman analyzer/IR is not given in the STP. Perform the identification test only from the composite sample of the material.
-
-
For API, received from the same group of companies (own other location). select the containers for sampling according to √n+1.
-
-
- If numbers of received containers are equal to or less than 5 then perform an identification test by NIR/Raman Analyzer/IR and take a sample for analysis and control sample from all containers but if the no. of the container is more than 5. Perform sampling according to √n+1.
-
- The analyst shall arrange the container one by one at a time under RLAF, which to be sampled and cut the tie lock of container and polybag with the help of cutting device.
-
- In the case of solvents. Perform sampling at the designated place of the solvent storage area.
-
- Take the sample from the top, middle and bottom for solid samples from every individual container.
-
- For liquid samples – Take the sample from the bottom of the container after properly mixing the liquid kept in the container with the respective sampling rod.
-
- The analyst shall check the collected sample of every container for any black/white/colored/ foreign particles, its color, any phase inversion in case of the liquid sample or any other discrepancy and record the observations in raw material sampling report (Annexure-1).
-
- If any black/white/colored/foreign particles, color change, any phase inversion or any other discrepancy is observed in the material during sampling then reject the material as per the decision of QC Head/Quality Head by filling the rejection note.
-
- Collect the sample quantity as per raw material sample quantity justification (Annexure- 5) g as follows:
-
-
Example: For Excipient
-
-
- Suppose 100 containers received for an A.R. No. and the required quantity of sample is 90g (for analysis and control sample).
-
- As per sample quantity justification then select the 11 containers for sampling according to √n+1 and take the approximately 10g sample from each selected container and make a composite sample and segregate the sample for following from composite sample.
-
-
- Microbiological test (if the material is to be analyzed for the microbiological test)
- Chemical test
- Control sample
-
-
-
Example: For API
-
-
- Suppose 15 containers are received in an A.R. No. and the required quantity of sample is 60g (for analysis and control sample)
-
- As per sample quantity justification, then after the spot identification test by NIR/Raman Analyzer, take approximately 13g sample from each container selected randomly as per √n+1, i.e from 5 containers and make a composite sample and segregate the sample from composite sample for following,
-
-
- Microbiological test (if the material is to be analyzed for the microbiological test)
- Chemical test
- Control sample
-
-
- In case of solvents take the required quantity of sample (in ml) by sampling device as per raw material sample quantity justification in a clean and dry glass bottle.
-
- After the segregation of the above sample in self-lock polybag or sterilized container (if the sample is to be analyzed for the microbial test) affix the respective label accordingly and return the leftover sample in to first sampled container and affix the leftover sample label on that container.
-
- After the sampling close the bags and containers properly with tie lock
-
- Put the “SAMPLED” stamp in green color on the quarantine label as shown below without disturbing the in house BAR CODE printed on the label.
SAMPLED
-
- Put signature and date the quarantine label of sampled containers.
-
- Place all the containers back to their designated place after completion of the sampling.
-
-
The Sampling of Psychotropic Raw Materials:
-
-
- QA shall make available the list of psychotropic materials to QC as per respective SOP.
-
- During the sampling of Psychotropic materials take the following precautions.
-
- Take the container from its cage rack by opening lock, in presence of RMS and proceed the sampling activity as per above procedure and note the tie lock number (if any) in sampling report .
-
- In absence of tie lock number, note as remark on sampling report and inform to Section Head then cut the tie lock and check the inner status label of the same and ensure that all the product information is the same as on the outer label.
-
- After sampling raw material, close the container properly with tie lock and note down the tie lock number on the sampling report as well as on the inner status label followed by signature and date.
-
- Similarly, close it with its outer lid and again lock with tie and note down the outer tie lock number on the sampling report and on the outer status label and transfer the container into respective location.
-
-
The Sampling of Hormone raw materials :
-
-
- QA shall make available the list of Hormonal samples to QC.
-
- Ensure. Only the properly trained workman and QC personnel shall work in the Sex Hormone (SH) area.
-
- The analyst shall ensure the availability of PPE’s in SH area sampling room.
-
- No personnel shall perform sampling in the Sex Hormone (SH) area without wearing PPE’s.
-
- During the sampling of hormonal material in the SH area. Restrict the entry in the sampling zone for girls.
-
-
The sampling of Raw Materials for Retest:
-
-
- A sampling of retest material to be done either on any day in the month of the actual due date or after.
-
- Where sampling is done on or after the due date, ensure that the previously approved labels are defaced and Metis generated “RETEST” label is attached to quarantine label.
-
- Sampling is to be done for both API and excipient according to Ön + 1 where “n” is the number of containers available in a lot or A.R. No. at the time of retesting as per procedure explained above.
-
- Take a sample quantity of retest material as per the quantity mentioned in raw material sampling quantity justification ( Annexure-5). and carry out the tests mentioned in the specification for retesting.
-
- Do not take the sample for unit identification test by IR from the containers of retest materials.
-
- Do not perform the Spot identification test of retest material by NIR/Raman Analyzer.
-
- Strikeout the initial sampled stamp is available on the container and put the new sampled stamp on each sampled container.
-
-
Additional sampling and testing of Raw Material:
- Perform the additional sampling as per the above procedure in case of container received in damaged conditions, event/rejection report/swab test, etc. after the recommendation.
-
-
- QA officer shall hold the impacted material physically as well as in Metis/Other relevant software.
-
- Designee from QA shall intimate QC for additional sampling and testing after approval of QA head & QC head through ‘Additional sampling Requisition Form’ (Annexure-4)
-
- Designee from QA shall fill the ‘Additional sampling Requisition Form’ in triplicate and mention the tests with the consultation of QC head and QA head which are to be carried out and two copies of this shall be submitted to QC department.
-
- Based on the tests mentioned, the designee from QC shall decide the sampling quantity and proceed for sampling and testing.
-
- The designee from QC shall fill the details in ‘Additional sampling Requisition Form’ and submit both copies to QA.
-
- Based on the analytical results and impact analysis, QA head shall mention the actions recommended in the respective column of ‘Additional sampling Requisition Form’
-
- Retain one copy and maintained by QC and another copy shall remain with QA.
-
-
Raw material sample quantity justification :
-
-
- Prepare the Raw material sample quantity justification as per Annexure-5 by the QC representative in excel sheet.
-
- Whenever there is any change in sample quantity, QC shall update Annexure-5.
-
- The version shall be given as YYYY/XXX,
-
- Where YYYY = Current Year (e.g 2020) and XXX = Serial No. starting from 001.
-
- QC person shall maintain the updated list by the concerned QC person with password protection.
-
- Made an excel sheet available in the specific drive of QC for reference to the section head or the concerned designee.
-
- Whenever there is any revision, the new version of the excel sheet shall be made available for reference and the old obsolete version shall be removed from a specific drive.
-
- The number/quantity of samples to be sampled shall be identified by the section head with the help of the Raw material sample quantity justification (Annexure-5).
-
- The specified quantity to be sampled shall be written by hand on the GIM by the section head to inform the analyst about the quantity to be sampled.
-
-
General Sampling Process For Microbiology Samples
- Perform the sampling of raw material for microbiological testing prior to collecting samples for other purposes.
-
-
- Sampling personnel shall wear clean, lint-free smocks, gowns or uniforms specified for the area where sampling is to occur.
-
- Wear sterile gloves and sanitized just before sampling.
-
- Sample the non-sterile material under a controlled environment, such as Laminar Air Flow hood or room with HEPA filtered air.
-
- Collect all microbiological samples using clean, sterile, implements, such as forceps, scoops, pipettes, and sample thieves.
-
-
Sanitize sampling tables and hoods using a validated sanitizing agent prior to use.
-
-
- Sanitize the exterior of the sampling implement packages and containers with a validated sanitizing agent prior to use with a clean lint-free wipe/cloth.
-
- Open packages and sample containers just before collecting the sample in order to minimize exposure to the environment.
-
- Hold the sample container lids such that the interior surface of the lid faces downward to avoid contamination falling into it.
-
- If the sample container lid cannot be held while sampling, it may be placed on a sanitized/sterilized surface with the interior lid surface facing down.
-
- Collect the microbiological samples for non-sterile dose facilities into sterile sample containers.
-
- Change the gloves between samples or sanitized with an approved sanitizer and allowed to dry.
-
- Wipe off the exterior of material containers with a validated sanitizing agent prior to opening. Ensure that the required contact time for the approved sanitizer has been achieved.
-
- Non-sterile raw materials and components, such as stoppers, caps, bottles, etc., shall be sampled in an enclosed environment that is temperature- and humidity-controlled. Sample tables, carts, scales, and booths shall be cleaned and sanitized prior to the collection of samples. Avoid leaning over open containers.
-
- Clean and sanitize the sampling areas after each sample is taken and after completing sample collection.
-
- Transport samples in a covered container to the appropriate Microbiology/ Environmental Monitoring Laboratory, as soon as sampling is completed.
7.0 ANNEXURES:
Annexure 1: Raw material sampling report
| Item Code | Received Date | ||
| Item Name | A.R. No. | ||
| Mfg. B.No. | Quantity | ||
| Manufacturer’s. Name | No. of container Received | ||
| Supplier’s Name | No. of container Sampled | ||
| Mfg. Date | Sample Qty. | ||
| Expiry/Retest. Date | Control sample Qty. |
Put √ mark as applicable):
Packing details: (Fiberboard Drums/HDPE drums/Plastic bags/Jute bags/Laminated paper bags/ M.S. container/S.S. container/Aluminum container/Shipper/Glass bottle)
Physical Inspection Check Points:-
| S.No. | Check Points | Remarks* |
| 1. | Check the material received from the approved vendor & its COA. | |
| 2. | Check the manufacturer’s COA for compliance with the specification. | |
| 3. | Check the status label of vendor & quarantine label. | |
| 4. | Check all details of the consignment received against GIM before sampling. | |
| 5. | Check the container for any physical damage. | |
| 6. | Check the seal integrity of the packing. | |
| 7. | Check the material for the presence of lumps during sampling. | |
| 8. | Check containers for observations such as black/white/colored/foreign particles during sampling. | |
| 9. | Check the silica gel bag or packet condition for its integrity, where required. | |
| Note : If discrepancies are observed , clearance shall be taken from QC Head or designee if further sampling is to be done :
Clearance given by (Sign/date) ________________________ |
||
*Note: Put (√) for complies and (X) for Does not comply in the remarks column.
Details of container Sampled : (Container No.) : __________________________
Note : Cleaned status label of the used sampling device should be attached at the back side of the report.
Cleaned status label attached by (sign/date) : …………………………………………..
Remarks (if any): ……………………………………………………………………………………………………..
Sampled By/Date: …………………… Checked By/Date: …………………………
Annexure – 2: Raw material sampling and cleaning log.
|
Sr. No. |
Date | Material Name | A.R No. | Sampling
Device ID. |
No. of container sampled | Sample Qty. | Nitrogen purging
done (Put √/or X) |
|
|
Sampling |
Cleaning | Done By | |||||||
|
Sampling room |
RLAF |
||||||||
| To | From | To | Solution Used | From | To | Solution Used | |||
Annexure – 3: Label For Microbiology Sample.
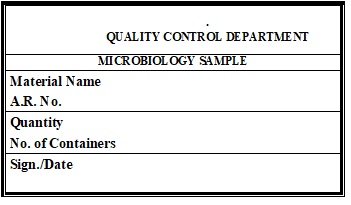
Annexure – 4: Additional Sampling Requisition Form.
| To: Quality Control Department | ||||
| From: | Date: | |||
We request you to take the sample of the material mentioned below for the necessary analysis and inform us of the result at the earliest.
| Item code : | ||||||||||
| Material Name : | ||||||||||
| Mfg. Name: | ||||||||||
| Supplier Name: | ||||||||||
| Mfg. Batch No. : | A.R.No. : | |||||||||
| Mfg. Date : | Exp. Date : | |||||||||
| Container(s) to be sampled:( x of y) | No. of container : | |||||||||
| Total Quantity: | ||||||||||
| Reference document name and number if any:_________________________
Reason for additional sampling & analysis: __________________________ |
||||||||||
Analysis Required (tests) :
|
||||||||||
| Prepared by
(QA) |
Approved by
(QA Head) |
Received by
(QC) |
QC Remarks
| Qty. Sampled: | Sampled by: | Analyzed by: | Checked by: |
| Remarks: Approved/ Not approved | |||
Annexure – 5: Raw Material Sample Quantity Justification
Make a table with following headings contents –
-
- Version No.
- Effective date
- Name of material
- Material received from
- Item code
- Description
- Solubility
- Other tests
- Total Quantity
- Qty. for analysis (in gm),
- Quantity for Control Sample (in gm),
- Quantity for retest (in g)
- Effective date
- Remarks
- Revision History
Sampling Device Issuance and Destruction Log (Annexure – 6)
Make a table with following headings contents –
-
- Sr. No.
- Name of sampling Device
- Sampling Device ID
- Issued by
- Destroyed by
- Destroyed on
- Reason for discardation
- Remarks
Annexure – 7: Label For Composite Sample.
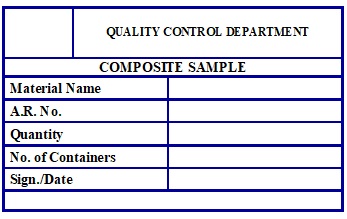
Annexure – 8: Label For Left – Over.
Copy above Label and replace “COMPOSIT SAMPLE” with “LEFTOVER SAMPLE”.
Label For Control Sample (Annexure – 9)
Copy above (ATTACHMENT – 7) Label and replace “COMPOSIT SAMPLE” with “CONTROL SAMPLE”.



Pingback: Quality Manual & Quality Policy in Pharmaceuticals - Guideline
Pingback: Good Laboratory Practices (GLP) - SOP & Guideline - CSTORE TRAINING
Pingback: Out of Specification (OOS) Handling Procedure - Guidelines - SOPs
Pingback: Good Laboratory Practices (GLP) - SOP & Guideline - Pharma Beginners