Standard Operating Procedure (SOP) and Guideline for preparation, approval, and revision of Site Master File (SMF). The Site Master File (SMF) Document shall contain specific information about the quality management policies, activities of the site, the production and/or quality control, pharmaceutical manufacturing operations, and any closely integrated operations at adjacent and nearby buildings.
Guideline for Preparation of Site Master File (SMF)
1.0 PURPOSE:
-
- The purpose of this SOP is to provide the procedure for preparation, approval, and revision of Site Master File (SMF) at the pharmaceutical drug manufacturing site.
2.0 SCOPE:
-
- The Site Master File (SMF) Document shall contain specific information about the
-
-
- Activities of the site,
-
-
-
- Production
-
-
-
- Quality Control,
-
-
-
- Pharmaceutical Manufacturing Operations, and
-
-
-
- Any closely integrated operations at adjacent and nearby buildings.
-
-
- If only part of a pharmaceutical operation is performed on-site, a Site Master File (SMF) shall only describe those specific operations (i.e., analysis, packaging, etc.).
3.0 REFERENCES:
-
- In House
-
- EUROPEAN COMMISSION – Volume 4 – Good Manufacturing Practice
-
- WHO Technical Report Series, No.961, 2011
4.0 RESPONSIBILITY- SITE MASTER FILE (SMF):
-
-
Quality Assurance (QA) Head/designee shall be responsible for-
-
-
- Creation of the Site Master File (SMF), review, approval of site master file (SMF).
-
- Implementing changes to the Site Master File (SMF) by utilizing the site Change Control process.
-
- Receiving the required data from the various departments at the site to create the Site Master File (SMF).
-
- Updating the Site Master File (SMF) periodically to ensure its compliance with applicable regulatory authorities.
-
-
Manufacturing head/designee shall be responsible for-
-
-
- Providing information to QA Head or designee for the Site Master File (SMF).
-
- Reviewing, providing recommendations, and approving the Site Master File (SMF).
-
- The Engineering/maintenance head/designee shall be responsible for
-
- Providing information regarding the facility Layouts/diagrams and other specifics required for the Site Master File (SMF).
-
- Providing recommendations and reviewing the Site Master File (SMF).
-
-
Quality Control (QC) head/designee shall be responsible for-
-
-
- Providing information regarding the list of laboratory equipment, and other specific documents required for the Site Master File (SMF).
-
- Providing recommendations and reviewing the Site Master File (SMF).
-
-
Quality Assurance Head/designee shall be responsible for-
-
-
- Ensuring the site has a Site Master File (SMF) which provides information about the production and control of manufacturing operations specific to the site.
-
- Reviewing, providing recommendations, and approving the Site Master File (SMF).
5.0 ABBREVIATIONS – SITE MASTER FILE (SMF):
-
- CCR: Change Control Record
-
- cGMP: Current Good Manufacturing Practices
-
- SMF: Site Master File
-
- DEFINITION:
-
- Batch (or Lot):
-
- A specific quantity of material produced in a process or series of processes so that it is expected to be homogeneous, to have uniform composition, character, and quality within specified limits, and is produced according to a master manufacturing order.
-
- Bulk:
-
- Any product that has completed all processing stages up to, but not including, final packaging.
-
- API –Active Pharmaceutical Ingredient:
-
- An ingredient intended to furnish pharmacologic activity or other direct effects in the diagnosis, cure, mitigation, treatment, or prevention of disease or to affect the structure or any function of the body; it does not include intermediates used in the synthesis of such an ingredient.
-
-
Corrective Action/Preventive Action:
-
-
- A concept with current Good Manufacturing Practice (cGMP) that focuses on the systematic investigation of root causes of unexpected incidences to prevent their recurrence (corrective action) or to prevent their occurrence (preventive action). (SOP for CAPA)
-
- Corrective Action:
-
- Action is taken to eliminate the causes of an existing nonconformity, defect, or other undesirable situation, in order to prevent a recurrence.
-
- Preventive Action:
-
- Action is taken to eliminate the cause of a potential nonconformity, defect, or other undesirable situation, in order to prevent occurrence.
-
-
cGxP:
-
-
- cGxP is a general term that stands for current Good “x” Practice (x = Clinical, Engineering, Laboratory, Manufacturing, Documentation, Pharmaceutical, etc.).
-
- The titles of these Good “x” Practice guidelines usually begin with “Good” and end in “Practice”. cGxP represents the abbreviations of these titles where “x” a common symbol for a variable, represents the specific descriptor.
-
- Change Control:
-
- A formal procedure for proposing, assessing, documenting, and approving changes that could affect the safety, purity, quality, or identity of the product, or could affect the validation of a process or testing methods.
-
- Change Control is a well-known cGMP concept that focuses on managing change to prevent unintended consequences. (SOP for Change Control Management)
-
-
Drug Product:
-
-
- A finished dosage form, for example, tablet, capsule, solution, etc., that contains an Active Pharmaceutical Ingredient(s) (API) generally, but not necessarily in association with inactive ingredients.
-
- The term also includes a finished dosage form that does not contain an active ingredient but is intended to be used as a placebo.
-
- Equipment:
-
- A device that operates either as a ‘stand-alone’ or combines several instruments or pieces of equipment to give an output.
-
- The equipment performs a unit operation or many unit operations.
-
- Parametric Release:
-
- A system of release that gives the assurance that the product is of the intended quality based on information collected during the manufacturing process and on the compliance with specific GMP requirements related to Parametric Release.
-
-
Quality Risk Management:
-
-
- Quality Risk Management is a systematic process for the assessment, control, communication, and review of risks to the quality of drug products across the product lifecycle. (SOP for Quality Risk Management)
-
-
Site Master File (SMF) :
-
-
- A document in the pharmaceutical industry, which provides information about the production and control of manufacturing operations.
-
- The document is created by a manufacturer.
-
- Validation Establishing documented evidence, which provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes.
6.0 PROCEDURE FOR PREPARATION OF SITE MASTER FILE (SMF):
-
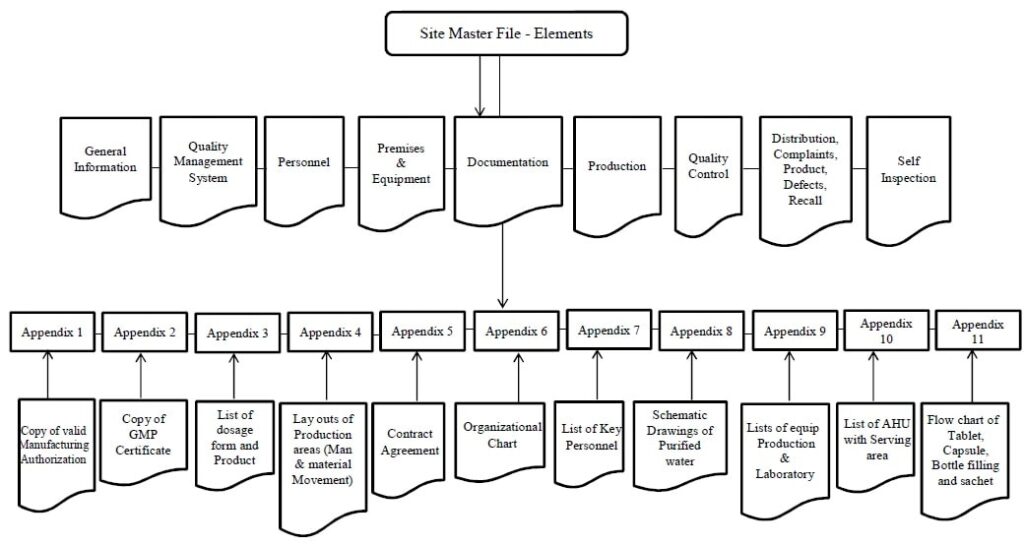
Site Master File (SMF) Key Elements:
-
- General Information of the Manufacturer
-
- Quality Management System of the Manufacturer
-
- Personnel
-
- Premises (Facility) and Equipment
-
- Documentation
-
- Production
-
- Quality Control (QC)
-
- Distribution, Complaints, Product Defects, and Recalls
-
- List of GMP inspections of the site within the last 5 years
-
- Self-Inspections
-
Site master file (SMF) appendix shall include the following information/documentation:
-
- Copy of Valid Manufacturing Authorization…………………………………………..Appendix 1
-
- Copy of Valid GMP Certificate………………………………………………………………Appendix 2
-
- List of dosage forms and molecules manufactured including the INN-names or common name (as available) of Active Pharmaceutical Ingredients (API) used…………………….. Appendix 3
-
- Layouts of Production area (Man & Material movement)…………………………Appendix 4
-
- Contract Agreement, List of contract manufacturers and laboratories including the addresses and contact information, and flow-charts of the supply chains for these outsourced activities…………Appendix 5
-
- Organizational Charts…………………………………………………………………………..Appendix 6
-
- List of Key Personnel……………………………………………………………………………Appendix 7
-
- Schematic drawings of the water system(s)…………………………………………….Appendix 8
-
- List of Major Production and Laboratory Equipment………………………………Appendix 9
-
- List of AHU with serving area……………………………………………………………….Appendix 10
-
- Flow chart of Tablet, Capsule, Bottle filling, and sachet……………………………Appendix 11
-
-
What is the Site Master File (SMF)?
-
-
- The document prepared by the manufacturer, containing specific and factual Good Manufacturing practices (GMP) information about the production and the control of pharmaceutical products at the site.
-
- The Site Master File (SMF) shall provide clear information regarding GMP related activities that can be useful in the general supervision and in the efficient planning and undertaking of GMP inspections.
-
- Contain adequate information but not to exceed 25-30 pages plus appendices.
-
- Simple plans outline drawings, or schematic layouts, are preferred instead of narratives.
-
- The Site Master File (SMF), including appendices, shall be readable when printed on A4 paper sheets.
-
- When submitted to a regulatory authority, the Site Master File (SMF) provides information on the manufacturer’s operations and procedures that can be useful in the efficient planning and undertaking of a GMP inspection.
-
- The SMF shall be prepared/reviewed once a year, preferably in the month of January of every year.
-
- The Site Master File (SMF) shall be part of documentation belonging to the Quality Management System and updated accordingly when necessary.
-
The numbering System for Site Master File (SMF) shall be as follows:
- XX/SMF/YYYY/ZZ
-
- Where:
-
- XX- stands for short name of company/site
-
- SMF- stands for Site Master File
-
- YYYY: Current Year (e.g. 2020)
-
- ZZ- stands for Revision number in ascending order starting with 00
-
- Any modifications thereafter shall be addressed as amendments to the SMF & reference change control details to be mentioned in the revision history.
-
- Any modification in the annexures shall be revised separately, no need to revise SMF.
-
- As and when SMF is revised, accordingly Annexures No. shall be revised with 00 revision suffix to the current revision no. of SMF.
-
- Wherever possible, simple plans, outline drawings, or schematic layouts shall be used instead of narrative.
-
- The Site Master File (SMF) shall be prepared by QA Head, Approved by the Quality head and Plant Head.
-
- Each section of the SMF shall start on a new page.
-
- The first approval page of the Site Master File (SMF) shall be signed by the QA head, Head-Quality, and Plant Head.
-
First Page of the Site Master File (SMF) shall contain the following headings:
- Name and Address of Site
-
-
- Title: Site Master File (SMF)
-
-
-
- Document Number, i.e. XX/SMF/YYYY/ZZ
-
-
-
- Effective Date and Next Review Date.
-
-
- A separate index shall be prepared for Annexures and Amendment(s) as per Annexure-2 & Annexure-3 respectively.
-
- The amendment can be in the form of printed formats, layouts, handwritten information, etc.
-
The Site Master File (SMF) shall be prepared on A4 paper using the following parameters:
| Parameter | Requirement | |
| Paper Size | A4 paper (210 X 297 mm) | |
| Paper Margin | Top 0.7’’ | Bottom 0.5’’ |
| Left 1.2’’ | Right 0.5’’ | |
| Header & Footer | Header : 0.8” Footer : 0.5” | |
| Paper Quality | White paper with Company Logo | |
| Header Text | <Company Name> Font: Times New Roman, Font Style: Bold, Font Size: 14, Alignment: Center |
|
| Manual Sub Heading | Font: Times New Roman, Font Style: Bold
Font Size: 12, Alignment: Center |
|
| Footer Text | Page X of Y | |
| * Adjust the header/footer in such a manner that the logo is not covered by Header text. | ||
- The Site Master File (SMF) shall be prepared with the following headings and details in the header.
| <Company Name>
<Company Address> |
|||
| Site Master File (SMF) | |||
| Document No.: | Title | Issue Date: | |
| Section No.: | Effective Date: | ||
| Supersedes: | Next Review Date: | ||
-
Where,
-
- Document No.: This is the unique alphanumeric identification number allotted to the Site Master File (SMF).
-
- Section: This shall be the serial number of that particular section. (Refer point no.)
-
- Supersedes: In case of revision of SMF, mention the SMF No. of the superseded SMF. In the case of new SMF, mention “NA”.
-
- Issue Date: The date on which the Site Master File (SMF) is approved by Quality Head, the same date shall be handwritten on all respective pages. The date format shall be DD/MM/YY.
-
- Effective date: The date on which the Site Master File (SMF) is approved by Factory Head, the same date shall be handwritten on all respective pages. The date format shall be DD/MM/YY.
-
- Next Review Date: Next Review date is the date before which the Site Master File (SMF) shall be reviewed. The date shall be handwritten in DD/MM/YY format.
-
- The Next review date shall be one year from the effective date, which shall be handwritten on all respective pages.
-
-
Title: This shall include the headings for that particular section.
-
-
- The footer for Site Master File (SMF) shall include page numbering in the form of Page X of Y, where X is the page number and Y is the total number of pages of Site Master File (SMF).
-
- The Site Master File (SMF) shall have the index page with the following details but not limited to :
| Section | Title | Page No. |
C1 |
General Information |
|
| C1.1 | Company’s Profile | |
| C1.2 | Name and Address of Site | |
| C1.3 | Manufacturing Activities | |
| C1.4 | Other Manufacturing Activity at Site | |
| C1.5 | Types of Product Manufactured | |
| C1.6 | Description of the Site | |
| C1.7 | Employment at Site | |
| C1.8 | Use of Out Side Technical Services | |
C2 |
Quality Management System |
|
| C2.1 | Release Procedure of Finished Products includes the following | |
| C2.2 | Management of Suppliers and Contractors | |
| C2.3 | Quality Risk management | |
| C2.4 | Annual Quality review | |
C3 |
Personnel |
|
| C3.1 | Organogram | |
| C3.2 | Key Personnel (Qualifications, Experience, and Responsibilities) | |
| C3.3 | Training | |
| C3.4 | Health Requirements for personnel engaged in manufacturing activity. | |
| C3.5 | Personnel Hygiene Requirement Including Clothing | |
C4 |
Premises & Equipment |
|
| C4.1 | Description of Manufacturing Areas (Man and Material Movement) | |
| C4.2 | Nature of Construction and Finishes | |
| C4.3 | Design Criteria For Ventilation Systems Schematic Diagram For HVAC and Dust Extraction System | |
| C4.4 | Special Area for the handling of highly toxic, hazardous, and sensitizing materials | |
| C4.5 | Brief Description of Water System and Other Utilities | |
| C4.6 | Planned Preventive Maintenance for Premises and utilities | |
| C4.7 | Description of Major Equipment for Production, Quality Control Laboratory & Engineering Equipment | |
| C4.8 | Planned Preventive Maintenance for Equipment | |
| C4.9 | Qualification, Validation, and Calibration | |
| C4.10 | Cleaning and Sanitation. | |
| C4.11 | GMP Critical computerized systems | |
C5 |
Documentation |
|
| C5.1 | Preparation, revision, and distribution of necessary documentation for manufacturing | |
| C5.2 | Other documentation related to product quality | |
| C5.3 | Additional Documentation | |
C6 |
Production |
|
| C6.1 | Brief description of production operation | |
| C6.2 | Arrangement for the handling of starting material, packaging material, bulk and finished product including sampling, quarantine, release, and storage | |
| C6.3 | A brief description of the arrangement for the handling of rejected materials and products | |
| C6.4 | Brief description of general policy for process validation | |
| C6.5 | Describe Policy for Reprocessing or Reworking | |
C7 |
Quality Control |
|
| C7.1 | Description of the Quality Control System and of the activities of the Quality Control Department & Procedures for the release of finished products | |
C8 |
Distribution, Product Defect, Complaint, and Product Recall |
|
| C8.1 | Description of Storage and Distribution Practices | |
| C8.2 | Handling of Product Defects, Complaints, and Product Recall | |
C9 |
Self Inspection |
|
| C9.1 | Description of Self Inspection Program | |
| C9.2 | Regulatory inspection | |
| List of Annexures | ||
| A separate index for Amendment |
-
C1 – General Information – Site Master File (SMF):
-
- C1.1 – Company’s Profile: (NMT 250 words/one A4 page)
-
- This shall include brief information about the organization, other sites, and information regarding the manufacturing activities at the company.
-
- C1.2 – Name and Address of Site:
-
- Name, and official address of the company’s manufacturing site contact person (s).
-
- Names, and street addresses of the site, buildings, and production units located on the site.
-
- Contact information for the manufacturing site, including twenty-four (24) hour telephone number, email address of the contact person in the case of product defects or recalls.
-
- Identification number of the manufacturing site such as Global Positioning System (GPS) details, or any other geographic location system,
-
- D-U-N-S (Data Universal Numbering System) Number (a unique identification number provided by Dun & Bradstreet) of the site and/or Facility Establishment Identifier (FEI), a unique identifier designated by FDA.
-
-
C1.3 – Manufacturing Activities – Site Master File (SMF):
-
-
- Authorized pharmaceutical manufacturing activities at the site include the following:
-
-
- Copy of the valid manufacturing authorization issued by the relevant Competent Authority in Appendix 1.
-
-
-
- If the Competent Authority does not issue manufacturing authorization, this shall be stated in the document.
-
-
-
- A brief description of manufacture, import, export, distribution, and other activities as authorized by the relevant Competent Authorities including foreign authorities with authorized dosage forms/activities, respectively; were not covered by the manufacturing authorization.
-
-
- The type of products currently manufactured on-site (list in Appendix 2) was not covered by Appendix 1.
-
- List of GMP inspections of the site within the last 5 years; including dates and name/country of the Competent Authority having performed the inspection.
-
- A copy of the current GMP certificate (Appendix 3) or other relevant references shall be included, if available.
-
- A brief description of Pharmaceutical manufacturing activities as licensed by the national authority.
-
- This shall also include the validity period for the manufacturing license issued by the national regulatory authority.
-
-
C1.4 – Other Manufacturing Activity at Site.
-
-
- This covers both pharmaceutical and non-pharmaceutical activities.
-
- C1.5 – Types of Products Manufactured – Site Master File (SMF):
-
- Type of actual products manufactured on the site and information about specifically toxic or hazardous substances handled, mentioning the way they are manufactured (in dedicated facilities or on a campaign basis). Product list to be attached as an annexure.
-
-
C1.6 – Description of the Site:(NMT 250 words/one A4 page)
-
-
- A short description of the site shall be explained under this clause.
-
- These shall include information about the location, immediate environment, area covered by the site, building facility details, size of the site, type of buildings, and their
-
- C1.7 – Employment at Site:
-
- The detailed information about the total number of employees engaged in the site shall be provided.
-
- This shall include any part-time employees also.
-
- The categorization of employees shall be done as mentioned below but not limited to:
-
-
- Production
-
-
-
- Quality Control
-
-
-
- Quality Assurance
-
-
-
- EHS
-
-
-
- IT
-
-
-
- P&A
-
-
-
- HR
-
-
-
- Storage & Distribution
-
-
-
- Technical & Engineering services
-
-
-
- Total of above
-
-
- Note: Include employees working only part-time on a full-time equivalent basis.
-
-
C1.8 – Use of Out Side Technical Services:
-
-
- Detail of use of outside scientific, analytical or other technical assistance in relation to manufacture and analysis shall be mentioned along with the name, address, telephone number, fax no, and brief outline of the activity being undertaken. (NMT 100 words/half an A4 page)
-
C2 – Quality Management System : (NMT 750 words/three A4 pages)
-
- The Quality Management System (QMS) of the company includes the following:
-
-
- Brief description of the quality management systems, and reference to the standards used for this system.
-
-
-
- Provide responsibilities related to the maintaining of the quality system including senior management.
-
-
-
- Provide information on activities for which the site is accredited and certified, including dates and contents of accreditations, names of accrediting.
-
-
-
The topics to be covered under these shall include but not limited to:
-
-
-
- Quality Policy and Objectives
-
-
-
- Introduction to Quality Division
-
-
-
- Role of Corporate Quality and Corporate Compliance Division
-
-
-
- Role of location Quality Assurance
-
-
-
- Management responsibilities
-
-
- Describe the elements of the QA system e.g. organizational structure, responsibilities, procedures, process, specifications, test methods, and other quality-related data collection.
-
- Internal Quality Audit Related: SOP for Internal Audit/Self Inspection
-
- Vendor Approval System
-
- Record if standards such as ISO 9001-9004 are used by the company to assess or audit the quality assurance system within the company or by the company to assess its suppliers.
-
- Brief description of Quality Risk Management methodologies.
-
- Scope and focus of QRM including a brief description of any activities which are performed at the corporate level, and those which are performed locally.
-
- Any application of the QRM system to assess continuity of supply should be mentioned.
-
- Short description of Quality by Design(QbD) and Continued Process Verification(CPV).
-
- A short description of the control strategy employs process analytical technologies (PAT).
-
-
C2.1 – Release Procedure for Finished Products includes the following:
-
-
- Provide a detailed description of qualification requirements, (education and work experience) of the Authorized Person(s) / Qualified Person(s) responsible for batch certification and release procedures.
-
- Provide a general description of batch certification and releasing procedure.
-
- Describe the role of Authorized Person/Qualified Person regarding quarantine, and release of finished products, and assessment of compliance with the Marketing Authorization.
-
- Describe the arrangements and roles between Authorized Persons/Qualified Persons when several Authorized Persons/Qualified Persons are involved.
-
- Provide a statement on whether the control strategy employs Process Analytical Technology (PAT) and/or Real-Time Release or Parametric Release.
-
-
C2.2 – Management of Suppliers and Contractors – Site Master File (SMF):
-
-
- Provide a brief summary of the establishment/ knowledge of the supply chain and the external audit program.
-
- Provide a brief description of the qualification system of contractors, manufacturers of active pharmaceutical ingredients (API), and other critical materials suppliers.
-
- Describe the procedure(s) in use to ensure that products manufactured are compliant with TSE (Transmitting animal spongiform encephalopathy) guidelines.
-
- Procedure(s) in use when substandard, counterfeit/ falsified products, bulk products (i.e. unpacked tablets), active pharmaceutical ingredients, or excipients, which are suspected or identified.
-
- Describe when the site utilizes outside scientific, analytical, or other technical assistance, in relationship to manufacture of product and analysis.
-
- Provide a listing of all contract manufacturers, and laboratories, including the addresses and contact information.
-
- Provide flow charts of supply-chains for outsourced manufacturing and Quality Control activities i.e.
-
-
- Sterilization of primary packaging material for aseptic processes,
-
-
-
- The testing of starting raw materials, etc., shall be presented in Appendix 4).
-
-
- Provide a brief overview of the responsibility-sharing between the contract giver and acceptor with respect to compliance with the Marketing Authorization.
-
-
C2.3 – Quality Risk Management (QRM):
-
-
- Provide a brief description of QRM methodologies used by the manufacturing site.
-
- Include the scope and focus of QRM including a brief description of any activities, which are performed at the corporate level, and activities, which are performed locally.
-
- Any application of the QRM system that is utilized to assess continuity of supply shall also be included.
-
- C2.4 – Product Quality Reviews:
-
- Brief description of methodologies used for Product Quality Reviews.
-
C3 – Personnel – Site Master File (SMF) (NMT 500 words/two A4 pages)
-
- C3.1 – Organogram:
-
- Provide an Organization chart showing the arrangements for
-
-
- Quality management,
-
-
-
- Production,
-
-
-
- Quality control,
-
-
-
- Engineering,
-
-
-
- Storage and distribution positions/ titles to be included in Appendix 5.
-
-
-
- Include senior management.
-
-
- The number of employees engaged in quality management, production, quality control, storage, and distribution respectively shall be included in the organogram and shall be attached as an annexure.
-
- C3.2 – Key Personnel (Qualifications, Experience, and Responsibilities):
-
- Brief details of academic qualifications, work-related qualifications and years of relevant experience shall be furnished.
-
-
C3.3 – Training:
-
-
- Brief details of the training program including induction and continuous training shall be explained along with the details as mentioned below but not limited to:
-
-
- How training needs are identified and by whom.
-
-
-
- Details of training relative to GMP requirements.
-
-
-
- State the form of training e.g. in-house, external, and how practical experience is gained.
-
-
-
- State how retraining needs are identified.
-
-
-
- Brief details of training records kept.
-
-
-
C3.4 – Health requirement for personnel’s engaged in manufacturing activity:
-
-
- A brief introduction to the medical check-up program shall be explained.
-
- These shall include but not limited to :
-
-
- Routine health checking of employees.
-
-
-
- Pre-employment and routine medical check-up based on the nature of work.
-
-
-
- System for reporting sickness or contact with sick people before working in a critical area and reporting back after illness.
-
-
-
- Additional tests/checks for employees working in sterile, cytotoxic, and hormone production area.
-
-
- C3.5 – Personal Hygiene Requirement Including Clothing:
-
- A brief description of washrooms and change rooms facilities made available for the employees shall be provided.
-
C4 – Premises and Equipment – Site Master File (SMF):
-
-
C4.1 – Description of Manufacturing Areas (Man and Material Movement):
-
-
- A simple plan or description of manufacturing areas with an indication of scale (architectural or engineering drawings are not required).
-
- A site plan highlighting the production areas shall be attached as annexure.
-
- If the products for different markets, i.e. for local, EU, USA, etc take place in different buildings on the site, the buildings shall be listed with destined markets identified.
-
- Man and Material movement shall be explained with the aid of suitable drawings.
-
- Attach the layout for each floor separately.
-
-
C4.2 – Nature of Construction and Finishes: (NMT 500 words/two A4 pages)
-
-
- This shall include a description about the below mentioned but not limited to:
-
-
- Material for the construction of the facility,
-
-
-
- Flooring,
-
-
-
- Electric supply,
-
-
-
- Ceiling,
-
-
-
- View panels,
-
-
-
- Coving details,
-
-
- Provide a simple plan of each production area along with the details of construction indicating the scale,
-
- All the areas shall be properly labeled/marked.
-
-
C4.3 – Design Criteria for Ventilation Systems Schematic Diagram for HVAC and Dust Extraction System: (NMT 500 words/two A4 pages)
-
-
- Give Salient features of ventilation systems.
-
- More details shall be given for critical areas with potential risks of airborne contamination (schematic drawings of the systems shall be attached as annexure).
-
- Classification of the rooms used for the manufacture of sterile products shall be mentioned.
-
- Room classification shall be given in accordance with the grading system outlined as per GMP.
-
- Schematic Diagram for HVAC and Dust Extraction System, Suitable diagrams/layouts shall be attached as annexure. (Preferably on A4 Paper only).
-
- For sterile product areas, a summary of the results of the most recent qualification/requalification shall be given.
-
- Following data shall be given:
-
-
- Design criteria e.g. specification of the air supply, temperature, humidity, pressure differentials, and air changes to rate, simple pass or recirculation (percentage),
-
-
-
- Filter design and efficiency,
-
-
-
- Provide the details of any alarms on the ventilation system,
-
-
-
- Show the PAO test and the point,
-
-
-
- Give the frequency of revalidation of the system.
-
-
- C4.4 – Special Area for handling highly toxic, hazardous, and sensitizing materials.
-
- Any Special Area for handling highly toxic, hazardous, and sensitizing materials shall be highlighted in the layout.
-
-
C4.5 – Brief Description of Water System and Other Utilities:
-
-
- A brief description of the water system along with schematic drawing shall be explained.
-
- This shall include but not limited to:
-
-
- The schematic drawing from the water source to final purified/ WFI water.
-
-
-
- The capacity of the system (maximum quantity produced per hour).
-
-
-
- Construction materials of the vessels and pipework.
-
-
-
- Specification of any filters in the system.
-
-
-
- If water is stored and circulated, the temperature at the point of return.
-
-
- The specification of the water produced:
-
-
- Chemical
-
-
-
- Conductivity
-
-
-
- Microbiological
-
-
- The sampling points and frequency of sampling
-
- Brief description of other relevant utilities, such as steam, compressed air, nitrogen, etc.
-
- C4.6 – Planned Preventive Maintenance for Premises and Utilities: (NMT 250 words/half an A4 page)
-
- The description of the planned preventative maintenance program and recording system shall be mentioned.
-
- This shall include (Site Master File – SMF) but not limited to:
-
-
- Planned preventative maintenance program.
-
-
-
- Written procedures and suitable reporting forms for maintenance and servicing.
-
-
-
- Whether maintenance routines that could affect product quality are clearly identified.
-
-
-
- Maintenance and Servicing of the Air Handling and Water Systems.
-
-
-
- “Maintenance” carried out by the manufacturer and “servicing” by an outside contractor.
-
-
-
C4.7 – Equipment: Description of Major Equipment for Production, Quality Control Laboratory & Engineering Equipment:
-
-
- Provide a listing of major production and control laboratory equipment with critical pieces of equipment identified included in appendix 8.
-
- This shall include but not limited to:
-
-
- The material of Construction of Equipment (e.g. AISI grade 316 stainless steel for product contact equipment).
-
-
-
- Are other materials used validated (e.g. polypropylene, chrome-plated brass, PVC, non-reactive plastic materials)?
-
-
- Whether equipment is designed for ease for cleaning.
-
- The general description of the equipment in production is to be furnished.
-
- In the quality control laboratory, only general descriptions such as pH meters, chromatographic equipment GC, HPLC with computer systems, particle size analyzers shall be provided.
-
- In microbiology use general descriptions such as incubators (temperature ranges) facilities for LAL testing, sterility testing, etc.
-
- Description of GMP critical computerized system (excluding equipment specific programmable logic controllers (PLCs)).
-
-
C4.8 – Planned Preventive Maintenance for Equipment: (NMT 250 words/one A4 page)
-
-
- A brief description of responsibility for maintenance and servicing of equipment,
-
- whether written procedures are available, are contract services from outside agencies taken, are records maintained for type and frequency of services, details of services repairs, and modifications.
-
-
C4.9 – Qualification, Validation, and Calibration:(NMT 750 words/three A4 pages)
-
-
- This shall include but not limited to:
-
-
- Brief description of the company’s general policy for qualification and validation.
-
-
-
- Briefly describe the re-validation policy.
-
-
-
- Areas covered under the validation program.
-
-
-
- An outline of process validation. (SOP for Process Validation)
-
-
-
- The system for the release for sale or supply of development and validation batches.
-
-
-
- The arrangements for computer validation, including software validation.
-
-
-
- Equipment/Instrument calibration policy and records kept.
-
-
-
C4.10 – Cleaning and Sanitation – Site Master File (SMF): (NMT 250 words/Half A4 page)
-
-
- This shall include but not limited to :
-
- Written specifications and procedures for cleaning, cleaning agents and their concentration, method of cleaning, and the frequency(i.e. Manual cleaning, automatic clean-in-place, etc).
-
- Change of cleaning agents from time to time.
-
- Validation of Cleaning Procedures.
-
- Method of evaluating the effectiveness of cleaning.
-
- Cleaning methods monitored routinely by chemical and/or microbiological methods.
-
- The cleaning methods (and their frequency) for the water supply system, air handling system and dust extraction system.
-
- C4.11 – GMP critical computerized systems:
-
- Description of GMP critical computerized systems (excluding equipment specific Programmable Logic Controllers (PLCs)).
-
C5 – Documentation – Site Master File (SMF): (NMT 500 words/two A4 pages)
-
- C5.1 – Preparation, revision, and distribution of necessary documentation for manufacturing:
-
- Description of the documentation system utilized at the manufacturing site (i.e. electronic, manual).
-
- When documents and records are stored or archived off-site (including pharmacovigilance data, when applicable):
-
- List types of documents/records; Name and address of storage site, and an estimate of document retrieval time required from the off-site archive.
-
- This shall include but not limited to:
-
-
- Documents such as Batch Manufacturing and Packing Records (BMR & BPR),
-
-
-
- SOPs,
-
-
-
- Specification,
-
-
-
- Analytical Test Procedures,
-
-
-
- VMPs,
-
-
-
- Scale Up reports,
-
-
-
- Validation protocol Reports,
-
-
-
- Technology Transfer documents,
-
-
-
- Qualification documents,
-
-
-
- Training material and records,
-
-
-
- Analytical Worksheets registers formats etc.
-
-
- Responsibility for the preparation revision and distribution of documents.
-
- Storage and handling of Master Documents.
-
- Brief Description for Documentation on:
-
- Product/Process Specifications
-
- Raw material specifications
-
- Packaging component specifications
-
- Standard process instructions including packaging
-
- Batch records including packaging
-
- Product quality review/Annual product review
-
- Analytical methods
-
- QA release procedures
-
- Document Control
-
- Retention Period for Documents
-
- Arrangements for any Electronic records / Backups
-
- If documents and records are stored or archived off-site, List of types of documents/records; Name and address of storage site, and an estimate of the time required retrieving documents from the off-site archive.
-
-
C5.2 – Other Documentation Related to Product Quality – Site Master File (SMF):
-
-
- This shall include but not limited to:
-
-
- Equipment specifications
-
-
-
- Specifications for disposables i.e. cleaning materials
-
-
-
- Standard operating procedure
-
-
-
- Quality Control Procedures
-
-
-
- Training procedures
-
-
-
- Computer program specifications
-
-
-
- Documentation control of process deviations
-
-
-
- Calibration and test documents
-
-
-
- Validation documents
-
-
-
- Reconciliation of bathes of raw materials, bulk product, major packing Components i.e. product-contact and printed material.
-
-
-
C5.3 – Additional Documentation :
-
-
-
- List and briefly explain the use of any additional standard documentation used routinely.
-
-
C6 – Production – Site Master File (SMF):
-
- Type of Products: In this section of the site master file (SMF), provide a reference to Appendix 1 or 2.
-
- The type of products manufactured includes the following:
-
-
- List of all dosage forms of both human and veterinary products, which are manufactured on site.
-
-
-
- List of all dosage forms for investigational medicinal products (IMP) manufactured for any clinical trials on-site, and when different from the commercial manufacturing, information of production areas and personnel.
-
-
- C6.1 – Brief description of production operations using, wherever possible, flow sheets and charts specifying important parameters but not limited to:
-
-
- Description of Manufacturing Operations.
-
-
-
- The operations capable of being carried out at the site with the existing facilities and specify the categories of medicinal products.
-
-
-
- If cytotoxic or hormone or radio-active substances are handled give details of the products.
-
-
-
- Explain the production operations using flow charts.
-
-
-
C6.2 – Arrangements for the handling of starting materials, packaging materials, bulk, and finished products, including sampling, quarantine, release, and storage.
-
-
- Identification of supplier’s lot number with the company’s lot number.
-
- Sampling plans.
-
- Status labeling e.g. by using labels or by computer.
-
- Issue of materials to manufacture and package.
-
- The control of weighing.
-
- Checking methods.
-
- Identification of materials to be used for manufacturing.
-
- Packaging shall include but not limited to:
-
-
- Release of bulk, semi-finished products, packing materials.
-
-
-
- Confirmation of identity and line clearance checks.
-
-
-
- In-process checks.
-
-
-
- Records of key parameters of manufacturing.
-
-
-
- Quarantine and release of finished products; compliance with the Marketing Authorization.
-
-
-
- The role of the Authorized Person(s) and/or Qualified Person(s).
-
-
-
C6.3 – Brief description of arrangements for the handling of rejected materials and products:
-
-
-
- Labeling of rejected materials.
-
-
-
- Storage in Secure and separate areas.
-
-
-
- Describe arrangements for sentencing the materials and their disposal.
-
-
-
- Maintenance of destruction record
-
-
- C6.4 – Process Validation:
-
- Brief description of the general policy for process validation.
-
- C6.5 – Reprocessing or Reworking:
-
- Describe the policy for reprocessing or reworking.
-
C7 – Quality Control – Site Master File (SMF):
-
- C7.1 – Brief description of the Quality Control activities carried out at the manufacturing site in terms of physical, chemical, and microbiological and biological testing, and the procedures for the release of finished products.
-
- Activities of the Quality Control Department shall include but not limited to:
-
-
- Briefly describe the activities of analytical testing, packaging, component testing, and microbiological testing.
-
-
-
- If the review of batch documentation and release of final documentation takes place in QC.
-
-
-
- Outline the involvement in the arrangements for the preparation, revision, and distribution of documents in particular those for specific test methods and release criteria if not mentioned elsewhere.
-
-
-
- Packaging Material and Raw material Testing.
-
-
-
- In-process and Finished Product Testing.
-
-
-
- Stability Testing.
-
-
-
- Microbiological Testing.
-
-
-
- Release System.
-
-
C8 – Distribution, Product Defect, Complaint, and Product Recall:
-
-
C8.1 – Description of Storage and Distribution Practices – Site Master File (SMF):
-
-
- Storage and Distribution shall include but not limited to:
-
-
- Description of Storage and Distribution Practices
-
-
-
- Details of warehouse security
-
-
-
- Environmental control
-
-
-
- Refrigerated storage
-
-
-
- How are the materials stored e.g. pallet racking?
-
-
-
- How is the status of products controlled e.g. by computer, by the label?
-
-
-
- Isolation of reject material securely
-
-
-
- Methods of distribution
-
-
-
- Dispatch order system to ensure first expire/first out and identification of the lot number
-
-
-
- Records of Distribution
-
-
-
- Types (wholesale license holders, manufacturing license holders, etc) and locations (EU/EEA, USA, etc) of the companies to which the products are shipped from the site.
-
-
- Brief description of the system to ensure appropriate environmental conditions during transit, e.g. temperature monitoring/control.
-
- Brief that retained records permit full batch traceability from the factory to the customer, in terms of the date of sale, customer details, and quantity dispatched.
-
- Arrangements for product distribution and methods by which product traceability is maintained.
-
- Provide a description of the procedures or measures taken to prevent manufacturers’ products to fall in the illegal supply chain.
-
-
C8.2- Handling of Product Defects, Complaints, and Product Recall:
-
-
- A brief description for the flow of complaints shall be explained to address the following but not limited to:
-
-
- Logging of complaints
-
-
-
- Classifying the complaints
-
-
-
- Investigating complaints
-
-
-
- Preparation and review of investigation reports
-
-
-
- For how long are complaints records kept
-
-
- Product Recalls: A brief description about the product recall system shall be explained which shall include but not limited to:
-
- Responsible persons for product recalls
-
- Details of written procedure which describes the sequence of actions to be followed including:
-
-
- Retrieval of distribution data;
-
-
-
- Notification to customers;
-
-
-
- Receipt/segregation/inspection of returned product;
-
-
-
- Investigation/reporting of the cause;
-
-
-
- Reporting corrective action;
-
-
-
- The person who notifies the Competent Authority of complaints and recall;
-
-
-
- The Competent Authority involved in complaints and the decision to recall;
-
-
-
- Effectiveness of recalls at below wholesale level;
-
-
-
- List of product recalled over the last two years;
-
-
-
- If none has been recalled, record-None.
-
-
C9 – Self Inspection – Site Master File (SMF):
-
- C9.1 – Description of Self Inspection Program:
-
- A short description of the self-inspection system utilized with focus on criteria used for selection of the areas to be covered during planned inspections, practical arrangements, evaluation of self-inspection, and follow-up actives.
-
- Documented procedures and records for the self-inspection system and the follow-up actions.
-
-
C9.2 – Regulatory Inspection – Site Master File (SMF):
-
-
- A brief description of inspections conducted by national authorities in the last five years.
-
- A brief description of inspections conducted by international regulatory authorities in the last five years, including date and name/country of the competent authorities.
-
- A copy of the current GMP certificates shall be attached.
-
-
Document and data control for Site Master File (SMF) :
-
-
- Appropriate information shall be supplied in form of Annexure and the reference of the same shall be made in the point.
-
- All the pages of the Annexure attached shall be numbered separately, i.e. they shall not be in continuation with the page numbering system of the Site Master File (SMF).
-
- The Site Master File (SMF) shall have a Revision history as below,
| Revision History | ||
| SMF No.: | Change Control No.: | Effective Date |
| Changes : | ||
-
- The Revision history shall be page numbered separately.
-
- The Revision history shall serve the purpose of Site Master File (SMF) reviews starting from the first revision 00 itself.
-
- The review of the Site Master File (SMF) shall be done within ± 30 days from the date of the Next Review.
-
- Any revision to be done to the Site Master File (SMF) in the existing year shall be in the forms of Amendments.
-
- The Obsolete copy of the Site Master File (SMF) shall be stamped as “OBSOLETE”, stored and destroyed as per document control SOP.
-
- If Revision is not required after review of the Site Master File (SMF), re-certify SMF for the validity of continuing usage as per respective SOP.
-
- QA shall issue a controlled copy of the site master file (SMF) to Factory Head for reference.
-
- Copy of Site Master File (SMF) required by any Regulatory / Any other Agency shall be forwarded through Head – Quality/ or designee by putting the stamp of UNCONTROLLED COPY.
-
- Uncontrolled Copy Issuance record of site master file (SMF) shall be maintained as per Annexure-4.
7.0 ANNEXURES – SITE MASTER FILE (SMF):
Annexure 1: Site Master File (SMF) WorkFlow.
Annexure 2: List of Annexure.
|
Sr. No. |
Annexures Number | Annexures Details | Total No. of Pages |
QA Head Sign/Date |
Annexure 3: Index for Amendment.
|
Sr. No |
Section No. of Site Master File (SMF) | Amendment No. | Effective Date | Total No. of Pages |
QA Head Sign /Date |
Annexure 4: Uncontrolled SMF Issuance Format.
|
Sr.No. |
Site Master File (SMF) No. | Issued To | Issued By/ Date |
Remarks |
***********************************************END***********************************************