Standard Operating Procedure (SOP) and Detailed Risk Assessment approach for Prevention of Cross Contamination, Mix-ups, and Microbial Contamination.
Prevention of Cross Contamination, Mix-Ups & Microbial Contamination
1.0 PURPOSE:
-
- The purpose of this procedure is to ensure that the manufacture of all finished drug products and Active Pharmaceutical Ingredients is accompanied by risk-based approaches and controls which are designed, implemented, maintained and monitored to manage the risk of Cross-Contamination, to a pre-defined, scientifically derived level of acceptability which meets current regulatory agency expectations and ensures patient safety and to prevent Mix-ups and Microbial Contamination.
2.0 SCOPE:
-
- This SOP provides a significant and holistic means to prevent and control Cross Contamination, Mix-Ups, and Microbial Contamination in Manufacturing and Packaging Facilities.
-
- In the event that local regulations exceed the requirements established within this SOP, the more stringent local requirements shall be applied.
3.0 REFERENCES:
-
- SOP for Quality Risk Management (Download)
-
- SOP for Corrective and Preventive Action (CAPA) – (Download)
4.0 RESPONSIBILITY – Prevention of Cross-Contamination:
-
-
Quality Assurance (QA) Team shall be responsible for –
-
-
- Reviewing and approving all documents related to control of cross-contamination, including protocols, studies, and SOPs.
-
- Ensuring all applicable risk reduction activities are processed through the CAPA / Quality Management System.
-
- Preparing and maintaining the quality risk management SOP for cross-contamination which includes initiating risk review activities as required.
-
- Ensuring all changes to manufacturing facilities, manufacturing processes, work procedures, and product mix are evaluated for their impact on the control of cross-contamination.
-
- Providing basic training to the operations staff on the control of cross-contamination.
-
- Providing performance data based on validations for use in risk analysis.
-
- Maintaining ADE monographs given by toxicologists.
-
- Maintaining and evaluating the equipment train while new production introduction/change in existing manufacturing area/equipment.
-
- Reviewing the risk as per review frequency and discussed with concern person for remedial action, if any.
-
-
Production / Warehouse / Quality Control / Personnel and Administration shall be responsible for –
-
-
- Participating in risk analysis exercises.
-
- Identifying and evaluating risks related to cross-contamination.
-
- Preparing and reviewing QRM documents (protocol/reports/SOPs) for control of cross-contamination as and when required.
-
- Investigating and evaluating the risks identified as a result of the technical implementation.
-
- Approving QRM documents as and when required.
-
-
Engineering shall be responsible for –
-
-
- Participating in risk analysis exercises.
-
- Preparing, reviewing, and approving the QRM documents.
-
- Implementing risk reduction measures that involve systems, facilities, utilities, equipment, and processes.
-
- Ensuring that the impact of any risk reduction measures is not detrimental to the systems, facilities, utilities, equipment, or processes.
-
- Maintaining the systems in good working order especially the HVAC system.
-
- Maintaining the calibration records for sensors, gauges, alarms, that control, monitor, and alarm systems for the management of the risk of cross-contamination.
-
- Advising if any of the systems, facilities, utilities, equipment, or processes are not performing as necessary to manage the risk of cross-contamination.
-
-
Environment, Health and Safety Team shall be responsible for –
-
-
- Participating in risk analysis exercises.
-
- Advising if any of the systems, facilities, utilities, equipment, or processes are not performing as necessary to manage the risk of cross-contamination.
-
-
QA head/designee shall be responsible for –
-
-
- Ensuring ADE values are available at the site.
-
- Reviewing and approving all documents related to control of cross-contamination.
-
- Ensuring that all the agreements with external agencies is available at the site.
-
- Leading the risk management team for the control of cross-contamination.
-
- Ensuring PDE values are available at the site.
-
- Reviewing and approving all documents related to control of cross-contamination.
-
- Ensuring implementation of SOP.
-
-
Toxicologists shall be responsible for –
-
-
- Providing the ADE Monograph reports to the site as given in the annexure3.
-
-
Operation Head/designee shall be responsible for –
-
-
- Approving all the documents related to control of cross-contamination.
-
- Leading the risk management team for the control of cross-contamination.
-
- Ensuring the implementation of SOP.
5.0 ABBREVIATIONS – PREVENTION OF CROSS CONTAMINATION:
-
- ADE: Acceptable Daily Exposure
-
- API: Active Pharmaceutical Ingredient
-
- CAPA: Corrective Action and Preventive Action
-
- CC No: Change Control Number
-
- CFT : Cross-Functional Team
-
- EMA: European Medicines Agency
-
- FDA: Food and Drug Administration
-
- FMEA: Failure Mode Effect Analysis
-
- HC: Hazard Category
-
- JP: Japanese Pharmacopoeia
-
- MDD: Maximum Daily Dose
-
- PDE: Permissible Daily Exposure
-
- QRM: Quality Risk Management
-
- RPN: Risk Priority Number
-
- STV: Safe Threshold Value
6.0 DEFINITION OF WORDS USED IN SOP FOR CROSS CONTAMINATION:
-
-
Combined Process and Risk Score:
-
-
- The combined process and risk score is the risk ranking of API used in the facility during a selected time period.
-
- Combined Process and Risk Score = Process Risk X Hazard Risk
-
-
Gradient Study:
-
-
- The gradient study is used to evaluate the risk via Mechanical transfer and airborne transfer.
-
- The study has two types of testing, air sampling, and settling plate.
-
- The settling plate measures actual sediment material, while the airborne measures the amount in the air (i.e. carrier) before sedimentation.
-
- Respectively, the settling plate is more accurate in evaluating the actual risk via all transfers, and airborne measurements enable determining airborne contribution.
-
-
Hazard Category:
-
-
- Classification of the materials based on their potential to cause harm, as determined by the PDE value.
-
-
Hazard Risk:
-
-
- The ranking of the hazard taking into account volumes and frequency of the manufactured and normalized by the PDE / ADE during a specific period.
Hazard Risk = Batch size API (kg) x Batches in period(per year)
PDE (mcg/day)
-
-
Permissible Daily Exposure:
-
-
- A daily dose of a substance below which no adverse effects are expected by any route, even if exposure occurs for a lifetime.
-
- This value is set by toxicologist based on clinical and other in-use data and is used to assess the risk of cross-contamination.
-
- This is also referred to as Acceptable Daily Exposure (ADE).
-
-
Process Risk:
-
-
- Process equipment is assigned a score based on the ability of the equipment to contain the product in the process.
-
- The process risk is the addition of all the risk scores for all the process steps.
-
- Process Risk = Sum of all equipment scores for the process for the product X Number of times equipment used.
-
-
Quality Risk Management:
-
-
- It is a systematic and scientific approach for the assessment, control, communication, and review of risks to the quality of drug products throughout their lifecycle.
-
-
Risk:
-
-
- The risk is a function of the hazard associated with the material and the potential exposure, which is the outcome of the controls.
-
- The risk is assessed by a combination of severity, occurrence, and detectability.
-
- Risk Priority Number:
-
- The Risk Priority Number, or RPN, is a numeric assessment of risk assigned to a process, or steps in a process, as part of Failure Modes and Effects Analysis (FMEA), in which a team assigns each failure mode numeric values that quantify the likelihood of occurrence, the likelihood of detection, and severity of impact.
RPN Calculation: Severity Score X Occurrence Score X Detection Score
-
-
Safe Threshold Value:
-
-
- The Safe Threshold Value is calculated from the Acceptable Daily Exposure and is the upper limit for statistical analysis used to determine the process capability and cleaning validation limits.
-
-
Toxicologist :
-
-
- Someone who possesses the requisite education, training, and experience in the field of toxicology and is recognized as qualified to apply appropriate consideration to all requested information.
-
-
Vulnerability (Product) :
-
-
- These are products with small daily doses per batch numbers.
Vulnerability = Batches in a period (per year)x 1000000
MDD in a batch
MDD in a batch = API per batch (in units/g)
The maximum daily dose of API (in units/g)
7.0 PROCEDURE FOR PREVENTION OF CROSS CONTAMINATION, MIX UPS, AND MICROBIAL CONTAMINATION:
-
Quality Risk Management Process to Assess and Control Cross-contamination Risk:
-
- When different pharmaceutical substances are produced in the same facility the potential for cross-contamination and mix-up always exists.
-
- Quality risk management shall be used to assess and control cross-contamination risk presented by the products manufactured.
-
- The risk assessment shall evaluate the potential risks associated with the cross-contamination of one product within another.
-
- High-risk products and high vulnerability products within the facility shall be identified based on risk management tools.
-
- The risk assessment shall consist of the identification of the hazards, analysis, and evaluation of risks associated with exposure to those hazards.
-
- The risk assessment undertaken to control the risk of cross-contamination must be a scientific and risk-based process that is comprised of the elements depicted under workflow.
-
- The level of effort and detail applied to the risk management process can and shall vary based on the perceived risks.
-
-
Results from the Quality Risk Management process shall be the basis for determining:
-
-
-
- The criteria used for decision making and establishing the control strategies used to manage the risk of cross-contamination.
-
-
-
- The necessity for and the extent to which facilities and equipment shall be shared, segregated, or dedicated to a particular product or product family.
-
-
-
- The extent of administrative, procedural, and technical controls required to manage risks for cross-contamination.
-
- Initiation of Assessment of Risk of Cross Contamination, Mix UPs, and Microbial Contamination:
-
QRM Team Selection:
-
-
- A cross-functional team shall be assembled to perform the Quality Risk management for cross-contamination as per the flowchart mentioned under workflow.
-
- Team members shall include (but need not be limited to) stakeholders in the area of:
-
-
- Development,
-
-
-
- Engineering,
-
-
-
- Validation,
-
-
-
- Product safety,
-
-
-
- Microbiology,
-
-
-
- Technical services,
-
-
-
- Manufacturing,
-
-
-
- Quality unit,
-
-
-
- Owner,
-
-
-
- Operator and
-
-
-
- Facilitator.
-
-
- Team members shall have appropriate knowledge, skill, authority, and expertise necessary to correctly assess and quantify the risk in a team environment.
-
-
QRM Planner:
-
-
- The planner for QRM activity shall include deliverables with action owners and target completion dates.
-
- Review the QRM activity periodically with the planner for its deliverables.
-
-
Risk Assessment:
-
-
- As per definition, risk assessment is a systematic process of organizing information to support a risk decision to be made within a risk management process.
-
- It consists of the identification of risk, analysis, and evaluation of risks associated with exposure to those hazards.
-
-
Risk Identification:
-
-
- The risk of the API is a constant that is characterized by an Allowable Daily Exposure (ADE) or Permissible Daily Exposure (PDE) value that is generated by toxicologists from clinical data, regulatory filings, market surveillance information, package inserts and other literature available.
-
- The ADE is the basis for a robust scientifically defensible strategy in managing the risk of cross-contamination.
-
- The severity of the risk is dependent on the primary compound ADE values.
-
- Determination of the ADE by the toxicologist.
-
-
Risk Analysis:
-
-
- Perform the analysis in three main phases to determine where exposure may occur and provide information to enable the ranking of potential risks.
-
-
- Completion of product assessment.
-
-
-
- Compliance review to current containment approach.
-
-
-
- Completion of site risk assessment according to FMEA.
-
-
Product Assessment for evaluation of cross-contamination risk:
-
- The first step of risk analysis is to understand which products pose the greatest risk and which are the most vulnerable to contamination.
-
- The completion of the product matrix comprises the assessment and evaluation of the gathered data related to the site, manufacturing, and packaging processes.
-
-
List of all product currently approved or being developed that are manufactured and packaged by the site including at a minimum:
-
-
-
- Products parameters
-
-
-
- Product code number
-
-
-
- Corresponding API (s)
-
-
-
- PDE Value
-
-
-
- % of API (If applicable)
-
-
-
- Batch size
-
-
-
- Maximal Daily Dose (in same units as batch size)
-
-
-
- Projected batches manufacturing frequency. If available, include a projection for 2 years.
-
-
-
- Maximum daily dose limit.
-
-
-
- Manufacturing process steps
-
-
-
- The qualitative risk score value of the manufacturing process.
-
-
-
The matrix summary report shall provide the following details, but not limited to:
-
-
-
- Material with the lowest PDE
-
-
-
- Product with lowest MDD/SBS ratio
-
-
-
- % of moderate hazard materials/products (PDE<100mcg) and of high hazard materials (PDE<1mcg)
-
-
-
- % of moderate hazard batches (PDE<100mcg) and of high hazard materials (PDE<1mcg)
-
-
-
- Highest process risk process
-
-
-
- Highest Volume/hazard value
-
-
-
- Combined risk (Highest)
-
-
-
- Highest Vulnerability
-
-
- The completion of the site matrix comprises the assessment and evaluation of the gathered data related to the site, manufacturing, and packaging processes.
-
- The matrix is designed to provide the data on the products produced, their hazards, characteristics of API, volumes manufactured, frequency of manufacture, processes used, risks, and vulnerabilities.
-
- From this master matrix, the products with the greatest risk and vulnerabilities are identified.
-
- The output of the Product Matrix must be used as an input for several other risk management tools including, but not limited to, the Failure Mode and Effects Analysis (FMEA) and Gradient Test.
-
- The outcome of the assessment and discussion of the product should be summarized as a chapter in the cross-contamination management report or as a separate document.
-
Compliance review to current cross containment approach:
-
- The approach and controls for the prevention of Cross-contamination should be detailed.
-
- The approach should address specifically each one of the pathways to prevent risk to adjacent products (physical barrier – segregation by place) and to the following products (time barrier – segregation by time).
-
- Each one of the control systems identified should be reviewed to ensure compliance of the design in each of the rooms to the containment approach.
-
- Example of the system to review: cleaning instructions, airlocks array, and pressure cascade, flows, gowning and gowning locations, dust collection, etc.
-
- The containment capability shall be defined for each product/material type based on the system and containment controls used for handling the respective product/material type.
-
- The outcome of the containment approach, control systems capabilities, and system review outcome, should be summarized as a chapter in the cross-contamination management report or as a separate document.
-
- The preexisting information required to be compiled in advance of performing the analysis includes:
-
-
- A complete list of compounds to be processed (including corresponding ADE’s/PDE’s).
-
-
-
- Facility drawings – including HVAC zoning and pressure cascades.
-
-
-
- Flow diagrams for people, products, and equipment.
-
-
-
- Historical data – including incident reports, recalls customer complaints, shop floor observation, failure investigations, cleaning data, and other test data such as gradient studies.
-
-
Risk Evaluation:
-
- FMEA (Failure Mode and Effects Analysis) is a qualitative and quantitative risk management tool used to help categories and prioritize the risks.
-
- The output of the FMEA is a risk ranking based on risk priority numbers (RPNs) that are then used to determine if the risk is acceptable or needs further action (reduction or additional data gathering).
-
- The FMEA provides a methodological approach for identifying risks of the production process in detail and the ranking of the RPN which identifies the impact of that risk.
-
-
The completion of the site FMEA comprises of the following:
-
-
-
- Identify high-risk products from the Site Matrix.
-
-
-
- Describe the product manufacturing stages and activities from raw materials receipt to final product dispatch.
-
-
-
- Each stage shall be analyzed based on the corresponding high-risk product identified in this stage.
-
-
- At each step of the manufacturing process, failure identification is initiated with a detailed description of the manufacturing step and the critical systems used in the process step.
-
- Identify modes of exposure for each potential failure. The following modes of exposure shall be reviewed.
-
Mix- Up:
-
- Mix-up may occur when any of the following items occur but not limited to:
-
-
- The wrong API is used in the process.
-
-
-
- Dirty equipment is used instead of clean equipment.
-
-
-
- The wrong dedicated part is used.
-
-
-
- The wrong label is placed on the container.
-
-
- To assess the risk of the mix-up, flow diagrams are required for personnel, materials, equipment, and waste streams.
Note: Refer to Annexure 4 – Checklist for Evaluation of Mix-up.
-
-
Retention:
-
-
- Retention may occur when any of the following items occur but not limited to:
-
-
- The cleaning procedure cannot meet health-based limits.
-
-
-
- The analytical method does not have the sensitivity to detect the health-based limits.
-
-
-
- Cleaning procedures are not followed.
-
-
-
- Confirmation of visual cleanliness is not adequate (the defined limit is below the visual detection limit) and chemical analysis is not performed.
-
-
-
- The capability of cleaning procedure was not tested i.e. cleaning validation was not performed.
-
Note: Refer to Annexure 5 – Checklist for Evaluation of Cleaning/ Retention Aspects.
-
-
Mechanical Transfer:
-
-
- The mechanical transfer may occur when any of the following items occur but not limited to:
-
-
- When particulate is carried from one process area into another via portable equipment, containers, pallets, and wastes.
-
-
-
- When particulate is carried from one process area into another via personnel, owning.
-
-
-
- Materials carry over in the production room on non-product contact parts and surfaces.
-
Note: Refer to Annexure 6 – Checklist for Evaluation of Mechanical Transfer Aspects.
-
-
Airborne Transfer:
-
-
- Airborne transfer occurs when any of the following items occur but not limited to:
-
-
- An airborne particle from one process sediments onto nonproduct contact surfaces and gets re-entrained into the airstream and is deposited into another process.
-
-
-
- Pressure cascades, airlocks, and/or filtration must be in place to manage the risk of airborne transfer.
-
Note: Refer to Annexure 7 – Checklist for Evaluation of Airborne Transfer aspects.
-
- Risk Assessment of quality-related events shall be performed to classify the risk category.
-
- The level of risk shall, in turn, help in prioritization of investigation, and finalization of strategy and CAPA used to resolve the incident/event.
-
-
The following three factors shall be considered when assessing the level of risk:
-
-
- Severity / Impact of Risk: Severity is fixed by the hazard (compound) and is dependent on the ADE when evaluating for cross-contamination risks.
-
- Probability of Occurrence: A rating factor used in risk analysis/assessment tools to indicate the likelihood of cross-contamination taking place considering plausible pathways. Each occurrence is determined by historical data or informed evaluation.
-
- Probability of Detection (State of Controls): A rating factor used in risk analysis/assessment tools to indicate how easily the exposure is detectable for any given occurrence.
-
- Assessment of Severity (S) / Impact of Event/Incident on cross-contamination:
-
- The event/incident which leads to cross-contamination shall be assessed for risk(s) to patient safety, identity, strength, purity, and quality of the product.
-
- The score rating may be performed as per SOPs for Quality Risk Management (refer to Annexure 9 as reference for score rating and criteria for the assessment of severity).
-
-
Assessment of Probability of Occurrence (O) of the Cause:
-
-
- The cause of the Incident/Event shall be reviewed to determine the ‘Probability of Occurrence’ in the future.
-
- The score rating may be performed as per the site SOPs for Quality Risk Management (refer to Annexure 9 as reference for score rating and criteria for the assessment of the probability of occurrence).
-
- Assessment of the “Probability of Detection (D)” (or “State of Control”):
-
- The QRM Team Leader / CFT shall assess the state of controls surround the incident/event and assign a score rating that may be performed as per the site SOPs for Quality Risk Management (refer to Annexure 9 as reference for score rating and criteria for the assessment of the probability of detection).
-
Risk Evaluation for Cross-Contamination:
-
- The output of a risk assessment is either a quantitative estimate of risk or a qualitative description of a range of risks.
-
- When the risk is expressed quantitatively, a numerical probability is used.
-
- FMEA is a widely used tool for Quality Risk Management.
-
- The other tools used are Fault Tree Analysis (FTA), Hazard Analysis and Critical Control Points (HACCP), Hazard Operability Analysis (HAZOP), Preliminary Hazard Analysis (PHA), etc.
-
- Risk evaluation included the comparison of the calculated RPN values produced in the FMEA with the established risk acceptance criteria so that a statement on the level of risk can be generated (refer to Annexure 9 as reference for RPN numbering and risk ranking).
-
Risk Control of Cross Contamination:
-
- The output of the Risk Assessment exercise shall be considered by the CFT to perform the Risk Control/Mitigation exercise to identify the action plans for control/mitigation of risk as immediate action based on risk evaluation.
-
- This may lead to the correction of processes, procedures, and practices to avoid the aggravation of the impact of the risk.
-
- Risk Control/Mitigation shall focus on the following questions:
-
-
- Is the risk above an acceptable level?
-
-
-
- What can be done to reduce or eliminate risks?
-
-
-
- What is the appropriate balance among benefits, risks, and resources?
-
-
-
- Are new risks introduced as a result of the identified risks being controlled?
-
-
- Control Measures/CAPA shall be considered for risk control/mitigation and shall be implemented using applicable procedures for the same.
-
- Such actions shall be prioritized to control/mitigate the risk or reduce it to an acceptable level.
-
Risk Reduction for Prevention of Cross-Contamination :
-
- Risk Reduction shall focus on processes for control/mitigation or avoidance of quality risk when it exceeds an acceptable level.
-
- It might include actions taken to mitigate the probability of harm.
-
- All control measures must be accompanied by mechanisms designed to detect the gap to the systems in place.
-
- As such, audible, visual, or otherwise recorded and monitored alarms must be utilized, where feasible, to assist in reducing risks to an acceptable level.
-
-
Risk Reduction may be achieved by (but not limited to):
-
-
- Improvement in the quality of the product by design – this may include improvement in the process, procedures, control measures, monitoring.
-
- Change of process and procedures.
-
- Revision of specification to stringent limits.
-
- Improvement of the periodicity of the measurement of parameters.
-
- Change in the frequency of calibration, qualification, validation, quality system internal audits in order to proactively identify the chances of the risk.
-
Risk Acceptance:
-
- Risk Acceptance is a decision to accept the identified & evaluated risk.
-
- It shall be a formal decision to accept the residual risk.
-
- For some types of harms, even the best quality risk management practices might not entirely eliminate risk.
-
- In these circumstances, it might be agreed that the optimal quality risk management strategy has been applied and that quality risk is reduced to an acceptable level.
-
- All items that are deemed acceptable risk will be monitored/ reviewed annually to ensure that the risk remains acceptable.
-
Risk Communication:
-
- Risk communication is the sharing of information about risk and risk management between the decision-makers and others.
-
- The parties can communicate at any stage of the risk management process.
-
- Risk communication must be incorporated into the following quality system:
-
-
- Training
-
-
-
- CAPA
-
-
-
- Change Control
-
-
- The output and result of the quality risk management process shall be appropriately documented by the process owner, reviewed by department heads, and approved by the management authorities.
-
-
The following is a listing of the minimum sections which should be included within each final report.
-
-
-
- Product Assessment:
-
-
-
- Risk Products
-
-
-
- % of high hazard products (PDE<100)
-
-
-
- Vulnerable products
-
-
-
- Plant/Lines STV value
-
-
-
- Product Matrix
-
-
-
Cross-contamination Approach:
-
-
-
- Description of a Cross-contamination prevention approach
-
-
-
- Summary of systems review
-
-
-
- Containment capabilities and limits
-
-
-
- Systems review forms
-
-
-
Risk Assessment:
-
-
-
- FMEA Spreadsheet
-
-
-
- FMEA supporting documents (cleaning verification assessments, etc.)
-
-
-
- Site QRMP-CC Team members
-
-
-
- Risk assessment process summary
-
-
-
Evaluation of Risk:
-
-
-
- Risk distribution according to the acceptable level.
-
-
-
Risk Control:
-
-
-
- High-Level Risk reduction plan (Table: CAPA number, risks ID, potential risk, action, due date and responsibility).
-
-
- Approved document.
-
Risk Review:
-
- A review of the QRM for cross-contamination and risk profile of the facility must occur when any change to the product, procedure, equipment, or system.
-
- It is recommended that the site SOP shall include a list of proposed changes.
-
- Based on risk, the review shall be done annually and a review report shall be prepared for further recommendation and action plan if any.
-
- SOP shall detail the scope of the review.
-
- The review shall include at a minimum: a review of deviations associated with cross-contamination, changes in the profile of the product, and CAPA follow-ups.
-
- Effectiveness of control measures / CAPA implemented to reduce the risk level shall be reviewed.
-
-
The CFT shall decide the effectiveness review criteria with respect to:
-
-
-
- What and Why to Review?
-
-
-
- How to Review?
-
-
-
- When to Review?
-
-
-
- Who will Review?
-
-
- The Risk Review Action Plan shall be handled through Action Item/Actionable, Tasks, or CAPA for Effectiveness verification. The Review/Closure shall be done as per site-specific procedures.
-
Prevention of Microbial (Cross) Contamination:
-
- A formal system of microbial contamination control (Environmental Monitoring Program) shall be established, implemented, and maintained within cleanrooms and associated environments.
-
- The formal system will assess and control factors that can affect the microbiological quality of the process and product.
-
- To assess and control the microbiological hazards, any selected system shall address the following principles:
-
-
- Identification of potential hazards to the process or product, assessment of the likelihood of occurrence of these hazards, and identification of measures of their prevention or control.
-
-
-
- Designation of risk zones and, in each zone determination of the points, procedures, operational steps, and environmental conditions that can be controlled to eliminate the hazards or minimize the likelihood of the occurrence.
-
-
-
- Establishment of limits to ensure control.
-
-
-
- Establishment of a monitoring and observation schedule.
-
-
-
- Corrective actions establishment to be taken when monitoring results indicate that a particular point, procedure, operation step, or environmental condition is not under control.
-
-
-
- Establishment of procedures, which may include supplementary tests and procedures, to verify that the chosen Formal System (Environmental Monitoring Program) is working effectively.
-
-
-
- Training procedures establishment.
-
-
-
- Establishment and maintenance of appropriate documentation.
-
-
Sources for Microbial Introduction:
-
- Internal and external sources exist which introduces the microbial contamination in manufacturing areas and eventually in the product.
-
- Below are listed the critical sources of microbial contamination.
-
-
Raw Materials:
-
-
- Some of the raw materials utilized in pharmaceutical formulations are based upon natural products that contain a high microbial load.
-
- The production processes for these raw materials do no eliminate all microorganisms.
-
- Therefore, they are not sterile.
-
- Testing must be performed to determine the quality of these materials.
-
- However, some of the manufacturing processes are designed to significantly reduce the number of microorganisms.
-
- Different types of bacteria commonly found in pharmaceutical raw materials are….
-
-
- Lactobacillus spp.,
-
-
-
- Pseudomonas spp.,
-
-
-
- Bacillus spp.,
-
-
-
- Escherichia spp.,
-
-
-
- Streptococcus spp.,
-
-
-
- Clostridium spp.,
-
-
-
- Agrobacterium spp., etc. and molds such as Cladosporium spp. and Fusarium spp.
-
-
-
HVAC System:
-
-
- HVAC systems in manufacturing facilities are built to minimize the survival, distribution, reproduction, and growth of microbes.
-
- Some of the microbial species commonly found in air samples in pharmaceutical environments are bacteria such as Bacillus spp., Staphylococcus spp., Corynebacterium spp. Common mold species are Aspergillus spp. And Penicillium spp.
-
-
Personnel:
-
-
- Microorganisms are part of the normal flora of the human skin and body.
-
- Therefore, operators and laboratory analysts are the major sources of contamination during manufacturing and testing.
-
- Some of the species living in the human skin are Staphylococcus epidermidis, Staphylococcus capitis, Staphylococcus hominis, Propionibacterium spp., Propionibacterium acnes, Micrococcus spp., etc.
-
- The normal flora for the human oral cavity is comprised of Streptococcus salivarius, Streptococcus mutans, etc. Molds can also be possible contaminants.
-
- Common molds from human flora are Trichophyton spp., Epidermophyton spp., Microsporum spp., etc.
-
-
Utilities:
-
-
- Water for pharmaceutical processes is further treated to minimize microbial numbers, endotoxin substances, and organic and inorganic compounds.
-
- The water with less organic compounds will have fewer microorganisms in it.
-
- Bacterial Species such as Pseudomonas spp., Alcaligenes spp., Stenotrophomonas spp., Burkholderia cepacia, Burkholderia picketti, Serratia spp., and Flavobacterium spp. Are commonly found in water samples.
-
- Other types of bacteria can also be present but when found, they indicate fecal sources of contamination.
-
- These bacteria are E. coli, Enterobacter spp., Klebsiella spp., Salmonella spp., Shigella spp., Clostridium perfringes, and Enterococcus spp.
-
-
Facility and equipment:
-
-
- Cleaning and sanitization of the equipment must provide a hostile environment for microorganisms to survive and grow.
-
- Bacteria such as Pseudomonas spp., S. epidermidis, Bacillus spp., etc. are commonly found in equipment.
-
- Molds are commonly found in walls and ceilings.
-
- Some of the mold species are Aspergillus spp., Penicillium spp., and Aureobasidium spp., etc.
-
- Other microbial species found are Taxeobacter spp., Flexibacter spp., Cytophaga spp., Ultramicrobacterium spp., Stenotrophomonas spp., Streptococcus spp., Sphingomonas spp., and Comamonas spp.
-
Measure for Prevention of Microbial Contamination: (SOP for Cross Contamination, Mix up)
-
- Below listed are minimum measures which shall be employed to prevent the microbial contamination of manufacturing area and products:
-
-
Raw Materials and Components:
-
-
- Raw materials shall be tested for incoming bioburden against documented acceptance criteria.
-
- RM including packing materials receipt and storage shall be done in a way which minimizes ingress and proliferation of microbial contaminants.
-
- All incoming material containers shall be checked for integrity of package, including tamper-evident seal where relevant, and for correspondence between the bill of lading, the purchase order, the supplier’s labels and approved manufacturer and supplier information.
-
- These receiving checks on each delivery shall be documented.
-
- Components and packaging materials shall at all times be securely stored off the floor to prevent contamination.
-
Heating Ventilation And Air Conditioning (HVAC) System: (SOP for Cross Contamination, Mix up)
-
- Areas shall be appropriately ventilated such that there is positive or negative pressure to prevent contamination to/from other areas.
-
- The pressure cascade shall be from most critical to least critical areas.
-
- Pressure differentials between rooms or areas shall be established using industry standards and guidelines issued by competent authorities (FDA, EMA, JP, etc.).
-
- Differential pressures between rooms or areas within the facility shall be monitored manually or through computerized building management systems as appropriate.
-
- Alert and action limits shall be established with defined procedures defining what responses shall be taken.
-
- If the pressure differentials are monitored continuously and do not meet established specifications, a local area alarm shall sound in the affected area(s) that is audible to the area personnel.
-
- Where airlocks are used they shall be interlocked, such that multiple doors cannot be opened simultaneously, causing a loss of differential pressure potentially resulting in cross-contamination.
-
- Aseptic process rooms must be maintained at higher differential air pressures than adjacent controlled areas.
-
-
Air filtration and air change rates shall be set to ensure that the defined cleanroom class is attained.
-
-
- Airflow over critical areas shall be uni-directional (laminar flow) at a velocity sufficient to sweep particles away from filling/closing area.
-
- HEPA filters shall be integrity (leak) tested at a minimum of once per year and twice per year for aseptic processing (ISO 5 / Grade A / Class 100).
-
- Ambient temperature and humidity shall not be uncomfortably high.
-
- The air pressure of the changing room shall be negative with regards to the manufacturing area corridor, but positive relative to external adjacent areas.
-
- Where possible ventilation dampers, filters, and other services shall be designed and positioned so that they are accessible from outside the manufacturing areas (service voids or service corridors) for maintenance purposes.
-
- Where appropriate, an impermeable barrier to prevent cross-contamination between two zones, such as closed systems, pumped or vacuum transfer of materials, shall be used.
-
- HVAC air distribution components shall be designed, installed and located to prevent contaminants generated within the room from being spread.
-
Personnel (Source of Cross Contamination, Mix up & Microbial Contamination):
-
- Access to production areas must be controlled to ensure entry is restricted to trained personnel only.
-
- A high level of personnel hygiene must be observed.
-
- All workers whose activities may affect the quality of the product must receive regular training, including hygiene instructions.
-
- Gowning requirements must be clearly defined and consistent throughout the facility for the same activity/area classification to prevent contamination.
-
- These requirements must be posted in the area of entry where items are donned.
-
- Every person entering the manufacturing area shall wear protective attire (over-garments, hair cover, beard, or mustache cover, overshoes) appropriate to the operations to be carried out.
-
- Direct contact shall be avoided between the operator’s hand and exposed products or any part of equipment that comes into contact with product.
-
- Employees having an apparent illness or open lesions that may adversely affect the safety or quality of pharmaceutical products shall be excluded from direct contact with components, drug product, packaging materials, and in-process materials until the condition is corrected or determined by medical personnel not to impact the safety or quality of the pharmaceutical product.
-
- Employees shall notify their supervisor if they have known health issues that could adversely affect the pharmaceutical products being made.
-
- Every individual entering the aseptic area shall be qualified for gowning through demonstration of proper gown technique and microbiological testing of the gown.
-
- Every individual shall be trained on the Aseptic technique training guide.
-
Utilities – (Source of Cross Contamination, Mix up & Microbial Contamination):
-
- Water and Pure Steam System:
-
- Water used in the manufacture of pharmaceutical products shall be suitable for the intended use.
-
- Wherever possible it shall be of pharmaceutical grade, microbiologically controlled and monitored.
-
- The system must be sanitized to prevent the formation of biofilms.
-
- Process Water, Purified Water, Water for Injection, and Clean Steam used in manufacturing and cleaning shall:
-
-
- Be validated/qualified for use.
-
-
-
- Meet applicable compendial / regulatory requirements for suitable purity (chemical, microbiological and particulates) for the intended use.
-
-
-
- Have generation, storage and distribution systems designed to prevent microbial growth, ensuring water quality can be maintained during dynamic and static operations.
-
-
-
- Have established microbial, chemical and particulates alert and action levels based on applicable compendial/regulatory requirements.
-
-
- Ensure employees are trained and understand how to react to alert and action level alarms.
-
- Be sampled, monitored, trended, and periodically reported for microbial and chemical contamination to the Quality Unit.
-
- Have deviations/incidents promptly notified to System Owners and the Quality Unit for investigation?
-
Compressed Gases / Air: (Source of Cross Contamination, Mix up & Microbial Contamination)
-
- Requirements apply to both purchased and in-house generated compressed gases.
-
- At a minimum, the microbiological and particulate purity of compressed gases/air shall match that of the environment into which it is introduced.
-
- Controls shall be in place to prevent the introduction of moisture, impurities, hydrocarbons, microbiological or other contaminants into the compressed gas/air systems.
-
- Testing of compressed gas/air for moisture, viable and non-viable particulates, hydrocarbons, etc. shall be performed periodically and documented.
-
- Compressed gas trend reports shall include a summary of excursions (alert/alarm).
-
Facility Design: (Source of Cross Contamination, Mix up & Microbial Contamination)
-
- The design of the facility floors, walls, ceilings and installation of the equipment/utilities shall facilitate effective inspection, cleaning and sanitization.
-
- Interior surfaces, e.g. floors, walls, ceilings shall be smooth, free from cracks and shall permit easy and effective cleaning.
-
- Windows/viewing panels shall be non-opening, flush with the wall panels, and properly sealed to prevent the collection of dust and microbial material.
-
- Pipework, ventilation and light points shall be designed to avoid the creation of recesses that are difficult to clean.
-
- To prevent potential cross-contamination drains in the processing areas shall be designed for ease of cleaning and provided with an air break or suitable device to prevent back-siphoning, when connected directly to a sewer.
-
- Sinks within the production areas shall be made of stainless steel.
-
- Depending upon the facility/product risk assessment results, Ventilated Cabinets, RABS (Restricted Access Barrier System), Isolators Systems, etc. must be used to achieve an absolute or partial barrier to contain microorganisms at their point of use.
-
- Airlocks and door barriers must be used to separate areas of unequal risk.
-
- All open-container processing shall be performed inside isolator / RABS (sterile products).
-
- All rooms and surfaces shall be maintained and monitored for viable and non-viable particulates and the facility must be recertified once per year for non-sterile manufacturing area and on a semiannual basis for aseptic processing (ISO 5 / Grade A / Class 100 or cleaner).
-
Equipment: (Source of Cross Contamination, Mix up & Microbial Contamination)
-
- Equipment cleaning/sanitization/sterilization procedures shall be in place to control microbial contamination.
-
- The material of construction of equipment shall withstand the effect of cleaning, sterilization or sanitization process and shall not develop rust or pitting.
Related: SOP for Equipment / Utility Cleaning and Sanitization
-
- Design of equipment shall facilitate ease in cleaning, sanitization and sterilization. Water or any cleaning agent shall not stagnate on the equipment surface.
-
Cleaning & Disinfection:
-
- Areas must be regularly cleaned and, where necessary, disinfected.
-
- A high level of cleansing and hygiene shall be practiced in every aspect of the manufacture of drug products.
-
- Disinfectant/sporicide rotation shall be practiced for effective control of microbial contamination.
-
- A suitable grade of cleaning agents shall be used to minimize health risks.
-
- Contact time, application, temperature, mechanical action, and the chemistry of the cleaning agents shall all be considered during the design of the cleaning process.
-
- Materials used for cleaning shall not come in direct contact with the product.
-
- Validation of cleaning practices must be carried out to provide evidence that the process is effective in controlling microbial contamination.
-
Laboratory Controls: (Prevention of Cross Contamination, Mix up & Microbial Contamination)
-
- Laboratories shall be designed to specifically meet the requirements of the operations carried out in them, e.g. containment for pathogenic biological or radioactive samples.
-
- There shall be sufficient space provided to avoid mix-ups and cross-contamination.
-
- Access to the Microbiology Laboratory shall be controlled and limited to authorized personnel.
-
- Laboratory work patterns shall be developed to ensure crossover is minimized or does not occur.
-
- Development laboratories and QC laboratories shall be separated from production areas.
-
- Preparation of microbiological growth media, testing, and decontamination of used growth media and materials shall be performed in segregated areas whenever possible.
-
- Multiple product types (non-potent as well potent) may be tested/developed in the same laboratory.
-
- Controls shall be developed through risk assessment, e.g., Man and Material movement shall be controlled to prevent cross-contamination through gowning practices and restricted access of lab personnel to sensitive manufacturing /packing areas.
-
- Sanitization agents used to disinfect the laboratory areas including benchtops, equipment, drawers, floors, walls, and ceilings as appropriate shall be qualified for efficacy in controlling the microbiological bioburden found in the laboratory area.
-
- The site Environmental Monitoring (EM) program shall include the Microbiological Laboratory where microbiological testing is performed and shall include areas where product testing could be significantly affected such as lab benches, sample receipt areas, walls, floors, equipment, etc.
-
Requirements: (Prevention of Cross Contamination, Mix up & Microbial Contamination)
-
- Quality Risk Management is a systematic and scientific approach for the assessment, control, communication, and review of risks to the quality of drug products/API throughout their lifecycle.
-
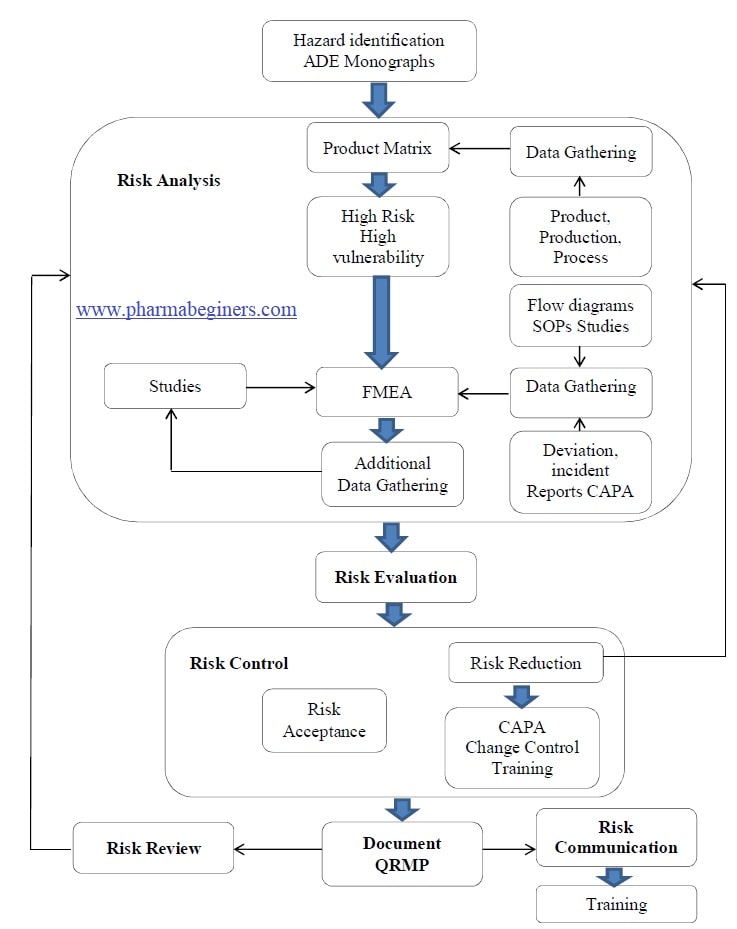
- Process Flow Diagram of Quality Risk Management outlines major phases of the risk management process including details of each phase and expected outcomes.
-
- Quality Risk Management shall encompass all products manufactured in the facility.
-
- The risk assessment shall evaluate the potential risks associated with the cross-contamination of one product within another.
-
- The initial phase of risk identification consists of the identification of and classification of compounds and potential hazards associated with the manufacturing of these compounds.
-
- Potential risks are identified based on the toxicological and pharmacological data gathered on the compounds.
-
- The severity of the risk for API is dependent on the primary compound Permitted Daily Exposure (PDE or ADE, Acceptable Daily Exposure) values. Derivation of a PDE includes both hazard identification and a dose-response assessment.
-
- Determination of PDE shall be done by an experienced toxicologist and this data shall be maintained centrally.
-
- Access to this data shall be provided on request to sites.
-
- The risk assessment undertaken to control the risk of cross-contamination must be a scientific and risk-based process that is comprised of the following elements.
-
-
- Health-based limits and product profile.
-
-
-
- 4 plausible pathways for cross-contamination.
-
-
-
- Risks analysis and assessment to control potential failures.
-
-
- The plant has to Creating, implementing, and managing systems to prevent and minimize the possibility for Cross Contamination, Mix-Ups, and Microbial Contamination.
-
- The plant is responsible for the implementation of this standard to meet the specific requirements.
8.0 ANNEXURES:
(SOP for Prevention of Cross Contamination, Mix up and Microbial Contamination)
Annexure 1: QRM Team for Cross Contamination Study :
| Sr. No. | Name of the Person | Designation | Department | ||||
Annexure 2: QRM Planner-Cum-Tracker for Cross Contamination Study “Template”.
Sr. No. |
Activity |
Deliverable |
Responsible Person |
Target Completion Date |
Actual Completion Date |
| 1 | Preliminary Preparations | ||||
| 2 | Appointment of QRM Leader and Committee | Team Member | |||
| 3 | Periodic meeting schedule | Schedule meeting | |||
| 4 | Permissions of project documentum | – | |||
| 5 | Training Day ( Introduction, SOP and Product Assessment) | Training form | |||
| 6 | Plant SOP or Protocol for Assessment | Approved SOP | |||
| 7 | Incorporation in change control procedure | Approved SOP | |||
| 8 | Procedure for changes evaluation | Approved SOP | |||
| 9 | Procedure for Annual Review | Approved SOP | |||
| 10 | Product Assessment | Product Matrix | |||
| 11 | List of Products and API ( Product Matrix) + Update on PDE gaps | Filled fields in the product matrix List of gaps for evaluation | |||
| 12 | API Parameters and PDE Gaps (Product Matrix) | Filled fields in the product matrix + Toxicological reports | |||
| 13 | Manufacturing Frequency (Product Matrix) | Filled field in the product matrix | |||
| 14 | Product Parameters (Product Matrix) | Filled field in the product matrix | |||
| 15 | Process Details (Product Matrix) | Filled field in the product matrix | |||
| 16 | Equipment Matrix | Filled field in the equipment matrix | |||
| 17 | Product Matrix Analysis + Report + Approved | Product Matrix + Analysis Summary Report | |||
Sr. No. |
Activity |
Deliverable |
Responsible Person |
Target Completion Date |
Actual Completion Date |
| 18 | Containment Approach | ||||
| 19 | Training Day ( Products Review + Containment Principles) | Training Form | |||
| 20 | Formulation of Containment Approach | Signed approach | |||
| 21 | Segregation in Time (Between Batch) | List Of systems for review Parameters to be reviewed | |||
| 22 | Segregation by space (Containment) | ||||
| 23 | System and procedures review: | ||||
| 24 | Mix- up
– Procedures and system |
Control systems description review table Containment Capability | |||
| 25 | Retention
– Cleaning Procedure – Cleaning Validation |
||||
| 26 | Airborne Transfer
– Room Matrix – Dust Collection system – Pressure Cascades – AHUs Drawing – Alert Time |
Control System Description Review Table | |||
| 27 | Mechanical Transfer
– Flow Diagrams – Gowning Procedure – Cleaning Procedure |
||||
| 28 | Gradient Study | ||||
| 29 | Settling Plate Study | Protocol + Report | |||
Sr. No. |
Activity |
Deliverable |
Responsible Person |
Target Completion Date |
Actual Completion Date |
| 30 | Airborne Sampling | Protocol + Report | |||
| 31 | Analyzing of Containment Capabilities | List of Manufacturing technology and its containment limit | |||
| 32 | Containment Approach report | Signed Report | |||
| 33 | FMEA Risk Assessment | ||||
| 34 | Training Day ( Risk Assessment of containment approach) | Training form | |||
| 35 | Process flow description and activities | Listed in FMEA | |||
| 36 | Identification of potential failures for each activity | Listed in FMEA | |||
| 37 | Initial risk Analysis – scoring according to current controls and historical data | Listed in FMEA supporting justification documents | |||
| 38 | High risks investigations | ||||
| 39 | Training Day ( Analysis of risks and studies design) | Training form | |||
| 40 | Cleaning Verification Assessment | Report | |||
| 41 | HVAC and dust collectors failure assessments | Report ( Can be included in gradient study) | |||
| 42 | Personnel Questionnaire | Report | |||
| 43 | Final FMEA scoring | FMEA Table | |||
| 44 | Risk Assessment summary report | Signed FMEA + Report | |||
| 45 | Risk Mitigation and Control | ||||
| 46 | Identification of approach gaps | List of gaps | |||
| 47 | Identification of risk for mitigation | List of Risks | |||
| 48 | Proposed corrective Action Plan | Table with proposed action for each item | |||
| 49 | Plan approval and CAPA | Approved Table CAPA evidence | |||
| 50 | QRM final report | Report |
Note: Contents in planner provided here area for reference only, the actual number of items may be an increase or decrease based on activities.
Annexure 3 : ADE/PDE Monograph.
Cover Page
The cover page shall include the name of the API and CAS number, authoring toxicologist, date of assessment, ADE value and any hazards identified.
Name of API
The name of the API including CAS number and any synonyms used.
Chemical Identity and Physico-Chemical properties
A description of the chemical structure, including a program, and the physic-chemical properties of the API.
Mechanism of Action and Intended Use
A description of the mechanism of action and inteded use of the API.
Animal Data
An overview of the animal studies conducted and their findings. Typical animal studies include:
- Acute and local toxicity
- Repeat dose toxicity
- Developmental and Reproductivity Toxicity
- Genotoxicity
- Carcinogenicity
Human clinical data
An Overview of the data obtained from human use. Typical information includes
- Dosages used in Clinical trials
- Adverse Reactions
- Susceptible Subpopulations.
Pharmacokinetics and Pharmacodynamics
A summary of the Pharmacokinetics and Pharmacodynamics information.
Derivation of the ADE/PDE
The ADE/PDE is calculated as follows:
PDE/ADE (mg/day) = NOAEL x B.W OR Lowest daily human dose (mg/day)
UFc x MF x PK
Where UFc is the composite uncertainty factor (UFA X UFH x UFS X UFL X UFD )
A detailed explanation on the derivation of the ADE/ADE including:
- Point of Departure (POD) or No Observed adverse effect Level (NOAEL)
- Uncertainty factor analysis including:
- UFA – Interspecies differences
- UFH – Interspecies differences ( Interindividual variability)
- UFS – Subchronic – to –chronic extrapolation
- Ufl – LOAEL –to – NOAEL extrapolation
- UFD – Database completeness
- MF – Modifying factor
- PK – Pharmacokinetic Adjustment
- Any additional factors deemed necessary by the toxicologist
Conclusion
A summary statement(s) on the derivation of the ADE
References
Provide a reference list of the list of all documents used to support the statements within the monograph
Signature
Provide the name and credentials of the authoring toxicologist as well as their signature. A current CV must be a record for the authoring toxicologist.
Note: This is a GMP controlled document and as such shall have a document number, revision date, and number, reviewed and approved by signatures and dates.
Annexure 4: Checklist for Evaluation of Mix-up.
|
Check Point |
Check |
||
1.0 Facility and Equipment Design/Controls |
Yes |
No |
|
| 1.1 |
|
||
| 1.2 |
|
||
| 1.3 |
|
||
| 1.4 |
|
||
| 1.5 |
|
||
| 1.6 |
|
||
| 1.7 |
|
||
| 1.8 |
|
||
| 1.9 |
|
||
| 1.10 |
|
||
| 1.11 |
|
||
| 1.12 |
|
||
2.0 Procedures/SOP |
|||
| 2.1 | Is there a procedure for the status labeling of equipment and facilities? Or if not, can the procedures and controls effectively prevent cross-contamination? | ||
| 2.2 | Is there a procedure for physical segregation, process flow, and security during material and product receipt handling, storage and staging? | ||
| 2.3 | Is there a robust line clearance procedure in place? | ||
| 2.4 | Is there a procedure for maintaining dedicated laundry? (i.e. keeping protective clothing inside an area where products with a high risk of cross-contamination are processed) | ||
| 2.5 | Identify areas where the existing procedures may fail or the consequences if the procedures are not followed. | ||
Annexure 5: Checklist for Evaluation of Cleaning/Retention aspects.
|
Sr. No. |
Check Point | Check | ||
| Yes |
No |
|||
| 1 | Check whether an acceptable level of retention or carryover is determined, taking into account the hazard(s) presented by the product and the nature and route of administration of the product that will be processed next in the equipment, unit or facility. | |||
| 2 | Check feasibility of the cleaning criteria | |||
| 2.1 | If a very low level of acceptable carryover is defined, are there analytical methods that will achieve the level of detection required? | |||
| 2.2 | If an aggressive cleaning agent (e.g. caustic or acids) is used what effects will this cleaning agent have on the equipment or materials of contraction? Will there be pitting or corrosion of surface with long term use? Will equipment parts become corroded or damaged over time (e.g. gasket material)? | |||
| 3 | Check practicability of the cleaning program | |||
| 3.1 | Would the effort required to perform a cleaning to the defined level be practical? | |||
| 3.2 | Would the facility need to be shut down for an extended period? | |||
| 3.3 | Would extra personnel be required to undertake the cleaning? | |||
| 3.4 | Would high volumes of solvent or other chemicals be required presenting a different risk scenario (e.g. exposure of workers to the cleaning materials, containment, and cost of disposal of solvent)? | |||
| Remarks: | ||||
Annexure 6: Checklist for Evaluation of Mechanical Transfer Aspects.
|
Sr. No. |
Check Point | Check | ||
| Yes |
No |
|||
| 1 |
Process |
|||
| 1.1 | Is the process closed? Can material escape the process and be transported around the facility via equipment, product, intermediates, supplies, and personnel moving through the facility? ( i.e. check whether process/room/GxP boundary is provided wherever needed) | |||
| 1.2 | Is there a system to change garments at the product boundary? | |||
| 1.3 | Is there a procedure for maintaining dedicated laundry (i.e. keeping protective clothing inside areas where products with a high risk of cross-contamination are processed? | |||
| 1.4 | Check surface –surface contact, e.g. scraping, smearing or wiping of process equipment | |||
| 1.5 | Check whether there are the repeated transfer of material to or from unit processes through breakable or poorly contained couplings | |||
| 1.6 | The requirement of the mobility of equipment/personnel due to use in the multiple areas, lesser the mobility, better the control to prevent cross-contamination | |||
2 |
Procedures |
|||
| 2.1 | Are procedures and/or control in place to minimize cross-contamination risk | |||
| 2.2 | Are procedures and/or controls in place that states equipment is to be wiped down prior to leaving the processing rooms? | |||
| 2.3 | Are there means to prevent the transfer from feet or equipment/trolley wheels? | |||
| 2.4 | Is de-contamination required to be carried out? If yes, is there a robust decontamination procedure in place? | |||
| 2.5 | Is there a procedure in place for waste isolation and handling contaminated rinsing water and soled gowning? | |||
| 2.6 | Is there a procedure for monitoring of working behavior to ensure training effectiveness and compliance with the relevant procedural control? | |||
| 2.7 | Is there a procedure in place for the separation of contract service provider activities? | |||
| 2.8 | Is there a procedure for recording and handling of spills/accidental events which may cause cross-contamination? | |||
| Remarks: | ||||
Annexure 7: Checklist for Evaluation of Airborne Transfer Aspects.
| Sr. No. | Check Point | Check | ||
| Yes | No | |||
| 1 | Process | |||
| 1.1 | Is the processing system a closed one or is the product exposed to the room environment during processing, transfer, or cleaning? Can material escape the process? | |||
| 1.2 | Are adequate engineering controls available to reduce the degree of a process’ openness? | |||
| 1.3 | Is the airflow pattern from ceiling to low level exhaust appropriate so that the operator and room entry is in the “clean zone” and the process is in the “dirty zone”? | |||
| 1.4 | Is there an appropriate gowning regimen to minimize the risk of cross-contamination by airborne transfer? | |||
| 1.5 | Is there an appropriate decontamination procedure (if needed) to minimize the risk of cross-contamination by airborne transfer? | |||
| 1.6 | Check whether process materials transmissible as aerosols must have certain physical properties to maintain a stable aerosol for a significant time period. | |||
| 1.7 | Check for risk of poorly sealed process equipment with pressure driving forces to promote undesired airborne transfer,e.g. positive pressure at source and/or negative pressure at receiving material positions. | |||
| 2 | Facility | |||
| 2.1 | Are the facility design aspects appropriate/adequate to control airborne transfer? (Interlock able airlocks, HVAC system, Pressure regimen, etc.) | |||
| 2.2 | Check whether the relative location of different process streams is adequate enough to minimize cross-contamination. | |||
| Remarks: | ||||
Annexure 8: FMEA Based Risk Assessment Examples.
| FMEA No. | |
| Reference Document No. |
| Risk ID | Department/Section | Room Use Suite Use | Process | Stage | Potential Failure |
| 1 | Dispensing | s-01/s-03s-04 | Weight to Batch | Filtration | Sedimentation into/ onto another product/process |
| Effect of Failure | Product | Potential Cause(s) | Occurrence | Justification for occurrence score | Current Controls |
| Airborne transfer | 10 | Filtration inadequate or Damage | 1 | To review shop floor observation/ Event Data | SOPs |
| Reference for current control | Detection | RPN | Recommended Action | Occurrence | Detection | RPN | Comments |
| 10 | 100 | Not Required | NA | – | NA | HEPA Of dispensing room damage risk |
Annexure 9: Risk Rating and Risk Evaluation.
Risk Assessment: Risk Assessment of quality-related events shall be performed to classify the risk category. The level of risk shall, in turn in prioritization and finalization of strategy and CAPA used to resolve the incident/ event.
Assessment of Severity (S) / Impact/ Incident on cross-contamination: Having determined that the event/incident may have a risk(s) on the safety, identity, strength, purity, and quality of the product.
The QRM team leader / CFT shall assign a risk rating as per table A below:
Table A: Severity/impact – Rating and Criteria
| Score | Severity (ADE mcg/day) | Criteria if cross-contamination occurs |
| 10 | ≥ 10 | Critical impact on patient safety |
| 7 | 11-100 | The potential impact on patient safety |
| 4 | 101 – 1000 | Moderate impact on patient safety |
| 1 | >1,000 | Low impact on patient safety |
Assessment of the probability of Occurrence (O) of the cause: The occurrence of cross-contamination is categorized as time-based or batch-based.
- Time-based occurrence: The events/incidents area reviewed for occurrence over a specific period of time (generally for one year).
- Batch based occurrence: The events/ incidents for the probability of cross-contamination area reviewed for product/batch-wise to study the impact on cross-contamination of the specific products.
The cause of the incident/event shall be reviewed to determine the probability of occurrence in the future.
The QRM team leader / CFT shall assign a risk rating as per table B below:
Table B: Probability of occurrence – Rating and Criteria
| Quantitative Raring | Time-Based Criteria (Events of Cross-contamination) | Batch based (Events of cross-contamination) |
| 10 | Occurred once or more per day | In every batch once or more |
| 7 | Once or more per month | In 50% or more of batches manufactured in the review period |
| 4 | Once or more per year | In 10- 49% of batches manufacture in the review period |
| 1 | Once in a year or more year | In ≤ 10% of batches manufacture in the review period |
- Assessment of the probability of Detection (D) (or, “State of control”):
The QRM team leader / CFT shall assess the state of controls surround the incident/event and assign a rating as per table B below:
Table C: Probability of Detection (State of Controls)- Rating and Criteria
| Score | Detection (Events of cross-contamination) |
| 10 | Can not be detected |
| 7 | Manual based system |
| 4 | Detected by analysis |
| 1 | Obvious or automatically alarmed |
Risk Evaluation:
Risk evaluation phase is the comparison of the calculated RPN values produced in the FMEA to the established risk acceptance criteria so that a statement on the level of risk can be generated.
- Determine the risk category / calculate the RPN values.
RPN Calculation: Severity Score X Occurrence Score X Detection Score
| RPN No. | Ranking | Production | Investigation | Remedial Action | Review |
| 343 – 1000 | Unacceptable Risk | Cease | Yes | Yes | Annually |
| 175 – 342 | High Risk | Continue | Yes | Yes | Annually |
| 100 – 174 | Significant Risk | Continue | Yes | Risk Control required | Annually |
| 1 – 99 | Acceptable Risk | Continue | No | No | Annually |
Annexure 10: WorkFlow.
*****************************************END******************************************


