This SOP provides guidance to evaluate the potential of the proposed new product for cross-contamination and assessment of the containment approach required to manufacture the new product at Manufacturing and Packaging Facilities.
For existing products, Quality Risk Management for Cross-contamination shall be performed as per SOP “Prevention of cross-contamination, Mix up and Microbial Contamination” for assessment of the potential of cross-contamination and implementation of controls to prevent the cross-contamination.
New Product Introduction & Risk Assessment
1.0 PURPOSE:
-
- The purpose of this procedure is to describe a systematic approach for the assessment of new product introduction for evaluation of cross-contamination risk at site and selection of containment approach for the manufacturing of the new product.
2.0 SCOPE – SOP FOR NEW PRODUCT INTRODUCTION:
-
- This SOP provides guidance to evaluate the potential of the proposed new product for cross-contamination and assessment of the containment approach required to manufacture the new product at Manufacturing and Packaging Facilities.
-
- For existing products, Quality Risk Management for Cross-contamination shall be performed as per SOP “Prevention of cross-contamination, Mix up and Microbial Contamination” for assessment of the potential of cross-contamination and implementation of controls to prevent the cross-contamination.
-
- In the event that local regulations exceed the requirements established within this SOP, the more stringent local requirements shall be applied.
3.0 REFERENCES:
-
- In House
4.0 RESPONSIBILITY– SOP FOR NEW PRODUCT INTRODUCTION:
-
-
Network strategy/corporate quality compliance/ shall be responsible for-
-
-
-
- Allocating a new molecule at the site with the consultation of site Quality Head and Plant Head.
-
-
-
- Reviewing the risk assessment reports of new product introduction.
-
-
-
- Reviewing the containment control available at the site.
-
-
-
Quality Assurance shall be responsible for-
-
-
-
- Participating in risk analysis activity to ascertain the separation category for a new molecule.
-
-
-
- Reviewing controls available at the facility to maintain containment.
-
-
-
- Ensuring all changes to manufacturing facilities, manufacturing processes, work procedures, and product mix are evaluated for their impact on the control of cross-contamination.
-
-
-
- Maintaining PDE monographs given by toxicologists.
-
-
-
- Maintaining and evaluating the equipment train while new production introduction/ change in existing manufacturing area/equipment.
-
-
-
- Reviewing the risk as per review frequency and discussed with the concerned person for remedial action if any.
-
-
-
- Ensuring all applicable risk reduction activities are processed through the Quality Management System.
-
-
-
Regulatory affairs shall be responsible for-
-
-
-
- Reviewing the regulatory requirements to manufacture a new molecule.
-
-
-
- Providing specific requirements for the manufacturing of new products/molecules.
-
-
-
- Reviewing controls available at the facility to maintain containment.
-
-
-
- Identifying and evaluating risks related to cross-contamination.
-
-
-
- Investigating and evaluating the risks identified as a result of the technical implementation.
-
-
-
Engineering shall be responsible for-
-
-
-
- Participating in risk analysis activity to ascertain the separation category for a new molecule.
-
-
-
- Reviewing controls available at the facility to maintain containment.
-
-
-
- Implementing risk reduction measures that involve systems, facilities, utilities, equipment, and processes.
-
-
-
- Ensuring that the impact of any risk reduction measures is not detrimental to the systems, facilities, utilities, equipment, or processes.
-
-
-
- Assisting in utility, facility, equipment qualification activities.
-
-
-
- Maintaining the calibration records for sensors, gauges, alarms, that control, monitor, and alarm systems for the management of the risk of cross-contamination.
-
-
-
- Advising and remediating if any of the systems, facilities, utilities, equipment, or processes are not performing as necessary to manage the risk of cross-contamination.
-
-
-
The formulation development department/technology transfer department shall be responsible for –
-
-
-
- Providing Technology Transfer Documents like Master Formula Card, Master Product Specification, Risk Assessment Report, Product Cleanability Data, etc. to location.
-
-
-
- Providing the specific requirements for the manufacturing of new product/ molecule like environmental conditions, safety requirements, gowning and operator protection requirements, etc.
-
-
-
Environment, Health, and Safety (EHS) shall be responsible for-
-
-
-
- Evaluating the new product/ drug substance for the safety requirements of operators.
-
-
-
- Participating in risk analysis activity to ascertain the separation category for the new molecule.
-
-
-
- Assisting in utility, facility, equipment qualification activities.
-
-
-
- Reviewing controls available at the facility to maintain containment.
-
-
-
- Advising if any of the systems, facilities, utilities, equipment, or processes are not performing as necessary to manage the risk of cross-contamination.
-
-
-
Quality Assurance Head/Designee shall be responsible for-
-
-
-
- Participating in risk analysis activity to ascertain the separation category for the new molecule.
-
-
-
- Reviewing controls available at the facility to maintain containment.
-
-
-
- Ensuring PDE values are available at the site.
-
-
-
The toxicologist shall be responsible for –
-
-
-
- Providing the PDE Monograph reports, for the product and all the highly potent intermediates, Key Starting Material (KSM), or other reagents/chemicals used, to site.
-
-
-
The pharmacovigilance (PVG) department shall be responsible for –
-
-
-
- Reviewing the safety data of a new molecule.
-
-
-
- Providing specific requirements for the manufacturing of new molecules.
-
-
-
Quality Head/Designee shall be responsible for –
-
-
-
- Leading the risk analysis activity to ascertain the separation category for a new molecule.
-
-
-
Quality Head/Designee shall be responsible for –
-
-
-
- Reviewing the risk assessment reports of new product introduction.
-
-
-
- Reviewing the containment control available at the site.
-
-
-
- Ensuring PDE values are available at the site.
-
-
-
- Ensuring the implementation of this guideline.
-
-
-
Plant Head/Designee shall be responsible for –
-
-
-
- Leading the risk analysis activity to ascertain the separation category for a new molecule.
-
-
-
- Reviewing risk assessment report of new product introduction.
-
-
-
- Reviewing the containment control available at the site.
-
-
-
- Ensuring the implementation of this guideline.
-
5.0 ABBREVIATIONS USED IN SOP FOR NEW PRODUCT INTRODUCTION:
-
- ADE: Acceptable Daily Exposure
-
- API: Active Pharmaceutical Ingredient
-
- CA: Containment Approach
-
- CC R No: Change Control Request Number (SOP – Change Control Management)
-
- CQ: Corporate Quality
-
- EHS: Environment Health & Safety
-
- HC: Hazard Category
-
- PDE: Permissible/Permitted Daily Exposure
-
- PVG: Pharmacovigilance
-
- SME: Subject Matter Expert
-
-
Biological Product:
-
-
- The term “Biological Product” means a virus, therapeutic serum, toxin, antitoxin, vaccine, blood, blood components or derivatives, allergenic product, protein (except any chemically synthesized polypeptide), or an analogous product, or arsphenamine or derivative of arsphenamine (or any other trivalent organic arsenic compound), applicable to the prevention, treatment, or cure of a disease or condition of human beings.
-
-
Containment Approach (CA):
-
-
- This is a group of recommended controls that are recommended with respect to the risk level associated with the material (hazard) and process properties (exposure).
-
-
Containment:
-
-
- The state achieved by enclosures with separation between manufacturing environment and manufacturing operation; the action of confining an entity within a defined space.
-
- Cross-Contamination:
-
- Contamination of a material or product with another material or product.
-
- Cytotoxic Materials:
-
- A material that directly damages or destroys cells and is used as chemotherapy to treat various types of cancer.
-
- The classification of material as cytotoxic is based on the mechanism of action.
-
-
Dedicated Block/Module in a multi-product facility :
-
-
- This is a defined unit, room, or area in a multi-product facility, dedicated to a specific product or product range, sufficient to address local risks associated with cleaning and/or other GxP concerns unique to the materials and processes under consideration by equipping it with its own air-locked personnel/material accesses and HVAC systems.
-
- Dedicated Building / Facility:
-
- Physically separate or segregated building with its own personnel/material accesses and HVAC systems, dedicated to processing steps associated with a specific product or product range.
-
- Dedicated Equipment:
-
- Equipment that is dedicated to a specific product or product range, and is identified accordingly.
-
-
Dedicated Item of Equipment:
-
-
- An equipment part (e.g., sieve, filter, feed chutes, compression tooling, filling heads, etc.) that is dedicated to a specific product or product range, and is identified accordingly.
-
- Consideration should be given to the cleaning, storage, and segregation of such items.
-
- Disposable Equipment:
-
- Equipment or small items of equipment that can be disposed of, and replaced, e.g., filling tubing, filters, or glassware.
-
-
Exposure Bands :
-
-
- Exposure, together with the hazard, is the factor that determines the risk.
-
- The exposure is derived from the process and controls.
-
- A low exposure band is associated with a lower risk.
-
- Hazard:
-
- A Hazard is a potential source of harm or adverse health effect on a person or persons, it addresses the harm derived in case of exposure.
-
- Hazard and exposure are the factors that determine the risk.
-
- It is an inherent property of the drug.
-
-
Hazard Category (HC) :
-
-
- Classification of the materials based on their potential to cause harm, as determined by the PDE value.
-
-
Permitted/Acceptable Daily Exposure (PDE/ADE):
-
-
- The PDE/ADE represents a dose that is unlikely to cause an adverse effect if an individual is exposed, by any route (e.g., intrathecal, inhaled), at or below this dose every day for a lifetime.
PDE (mg/day) = : _NOAEL x B.W OR Lowest daily human dose (mg/day)__
UFc x MF X PK
-
- NOAEL: No-Observed-Adverse-Effect Level (mg/kg/day)
-
- BW: Body Weight (kg)
-
- UFc: Composite uncertainty factors (UFA, UFH, UFS, UFL, UFD)
-
- UFA: Interspecies (1= since the human lowest dose is taken for calculation,
-
- If it is based on non – human NOAEL or LOAEL values then the value will be greater than 1)
-
- UFH: Intraspecies variability
-
- UFS: Sub-chronic to chronic extrapolation
-
- MF: Modifying Factor
-
- UFL: LOAEL to NOAEL extrapolation
-
- UFD: Database completeness
-
- PK: Pharmacokinetic Adjustment(s)
-
-
Penems:
-
-
- A β-lactam broad-spectrum antibiotic acts on cell walls, lysing bacteria in the resting phase.
-
-
Potent Drugs:
-
-
- The potency is a measure of drug activity expressed in terms of the ‘amount required for producing an effect of given intensity’.
-
- A highly potent drug evokes a larger response at low concentrations, while a drug of lower potency evokes a small response at low concentrations.
-
- It is proportional to affinity and efficacy.
-
-
Risk :
-
-
- Cross-contamination risk is derived from the hazard level of the materials and the potential exposure as determined by the process properties and containment controls.
-
- With stringent exposure controls, the risk of high hazard materials can be reduced to an acceptable level.
-
-
Sex hormones :
-
-
- A hormone that is produced especially by the ovaries, testes, or adrenal cortex and affects the growth or function of the reproductive organs or the development of secondary sex characteristics.
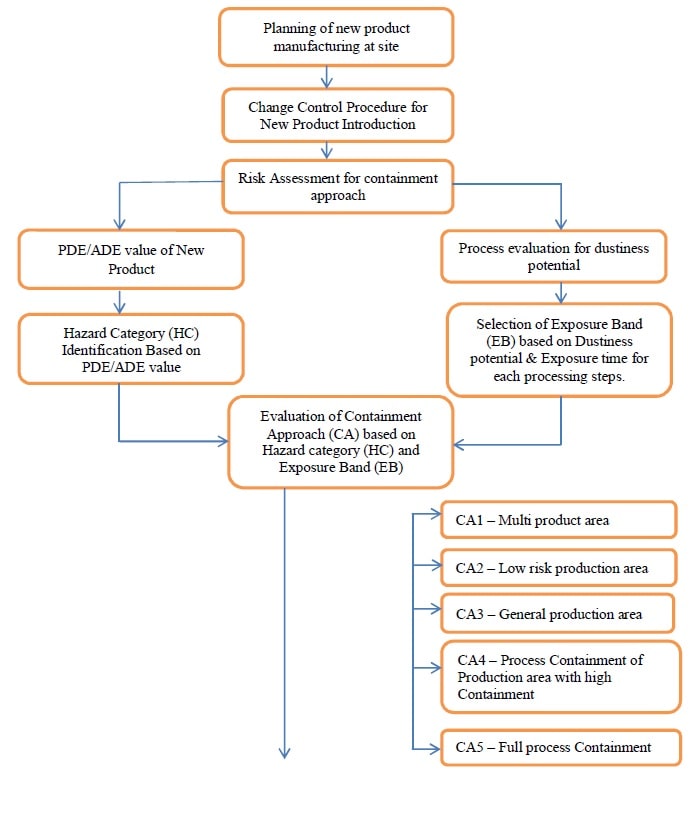
6.0 PROCEDURE FOR INTRODUCTION OF NEW PRODUCT:
-
Evaluation of new product/molecule:
-
- A new product/Molecule shall be evaluated for possible hazard based on its toxicological data.
-
- To identify acceptable risk, health-based limits (i.e. PDE) should be developed by an authorized toxicologist from:
-
-
- Toxicological and pharmacological data.
-
-
-
- Data that are submitted, as required, with Marketing Applications (i.e. New Drug Applications (NDAs), Biological License Applications (BLAs), or Marketing Authorization Applications (MAAs)) normally in Common Technical Document (CTD) format.
-
-
-
- Data from clinical trials.
-
-
Evaluation of containment requirement for new product/ molecule:
-
- R&D or Manufacturing shall follow the steps below to decide the separation category for the new product/molecule.
-
- The decision should be approved by SMEs from Manufacturing / QA / Engineering / RA /PVG / EHS / Technology Development /Technology Transfer (as required) and Responsible Person.
-
- Step 1: Dedicated facilities are required for manufacturing when a medicinal product presents a risk because:
-
-
- Scientific data from the toxicological evaluation does not support a controllable risk (e.g. allergenic potential from highly sensitizing materials such as beta-lactams).
-
-
-
- There is a specific regulatory requirement to handle the product in a type dedicated facility (Refer Annexure-1 for information about such requirements).
-
Note: That requirement is based on the markets that are supplied by the site and not the specific product.
-
- Step 2: For the products not falling in the criteria mentioned in the above Section, a comprehensive Risk Analysis as explained in the below Section(Preliminary risk analysis) shall be performed to ascertain the separation category.
-
Preliminary risk analysis:
-
- A Preliminary Risk Analysis shall be carried out to derive the product separation category.
-
- The cross-contamination risk level is a function of the hazard and potential exposure.
-
- The approach for the same shall include the following major steps (but not limited to):
-
- Step 1: Calculate Hazard of Impact of Cross-Contamination based on the PDE values of the materials and conclude. This classification addresses the pure harm potential and it is determined by the properties of the materials handled.
| Hazard Category (HC) | PDE mcg/day |
| HC 1 | ≥1000 |
| HC 2 | ≥100, <1000 |
| HC 3 | ≥10, <100 |
| HC 4 | ≥1,<10 |
| HC 5 | <1 |
-
- Step 2: Conclude the Exposure Band (EB) based on the properties of the process and the following table.
-
- The EB evaluates potential exposure due to the process.
-
-
Low EB indicates lower risk due to the lower potential exposure.
-
| Quantity of API Handled | Dustiness Potential | Exposure Time | ||
| Low
e.g. coated pellets/ tablets, closed capsules, and liquids |
Medium
e.g. wet solids, heavy granule, uncoated tablet |
High
e.g. Powder, sifting, milling, compaction, sampling, dispensing |
||
| Small (up to 1000 g) | Exposure Band 1 | 1 | 2 | Short-
Below 15 minutes |
| Exposure Band 1 | 2 | Exposure Band 3 | Long-
More than 15 minutes |
|
| Medium (up to 1000 kg) | Exposure Band 1 | 2 | Exposure Band 3 | Short –
Below 15 minutes |
| Exposure Band 2 | 3 | Exposure Band 4 | Long – more
than 15 minutes |
|
| Large (more than 1000 kg) | Exposure Band 2 | 3 | Exposure Band 3 | Short –
Below 15 minutes |
| Exposure Band 3 | 4 | 4 | Long – more
than 15 minutes |
|
-
- As the exposure level in a different step of the production may vary, the assessment should be done for each major production step (i.e. outcome may be that granulation requires a different containment approach then coating).
-
- For preliminary evaluation of a product, the highest exposure levels applicable for the process steps should be considered (i.e. the worst-case containment level required for at least one of the production steps).
-
Step 3: Evaluate the appropriate Containment Approach (CA).
-
- The containment approach shall be in compliance with the risk of cross-contamination.
-
- The Cross-contamination risk is derived from the potential hazard (as expressed in the Hazard Category), and potential exposure (as expressed in the Exposure Band).
-
- Based on the risk levels, the containment approach is determined.
| EB 1 | EB 2 | Exposure Band 3 | EB 4 | |
| HC 1 | CA 1 | CA 1 | Containment Approach 1 | CA 2 |
| HC 2 | CA 1 | CA 2 | Containment Approach 3 | CA 3 |
| HC 3 | CA 2 | CA 3 | Containment Approach3 | CA 4 |
| HC 4 | CA 2 | CA 3 | Containment Approach 3 | CA 4 |
| HC 5 | CA 3 | CA 3 | Containment Approach 4 | CA 5 |
-
- In order to establish a standard for product separation to prevent cross-contamination, the following types of separation categories are considered:
-
-
- Containment Approach 1 –
-
-
-
-
- Multi-product area.
-
-
-
-
- Containment Approach 2 –
-
-
-
-
- Low-risk production area
-
-
-
-
- Containment Approach 3 –
-
-
-
-
- General production area
-
-
-
-
- Containment Approach 4 –
-
-
-
-
- Process containment (recommended) or production area with high containment
-
-
-
-
- Containment Approach 5 –
-
-
-
-
- Full process containment
-
-
Note: The separation categories are explained in Annexure-II.
-
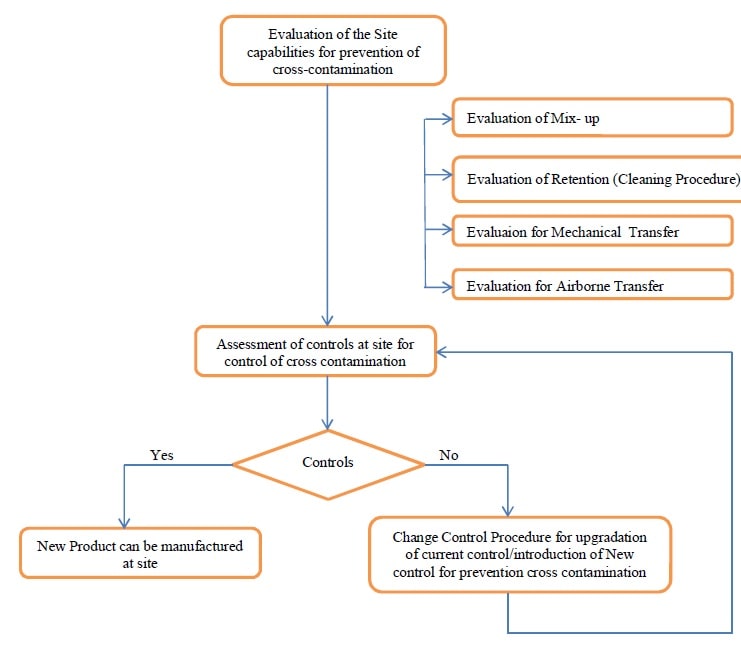
- After the determination of the Containment Approach for processing/manufacturing of new drug products or drug substances, evaluation of the capabilities shall be performed with respect to four (4) pathways of cross-contamination.
-
- The activity shall be managed through the change control process and impact assessment for new product introduction shall be performed using the checklist provided.
-
- The checklists provided are for reference purposes.
-
- The contents of the checklist can be designed considering the product requirement, prevailing system, and controls available at the site.
- Evaluate capabilities for handling the material based on the 4 pathways for cross-contamination:
-
-
Evaluate for Mix-up:
-
-
- Are procedures controls and facilities designed such that mix up is avoided?
-
- Refer to Annexure III – Checklist for evaluation of the potential for Mix-up based on Facility and Equipment Design Controls and Procedures/SOPs.
-
- List the gaps, identified based on the checklist, and the required control for the manufacturing of products.
-
-
Evaluate for Retention (cleaning procedures):
-
-
- Evaluate for each equipment item if the current cleaning procedure can meet the required criteria.
-
- Refer to Annexure IV – Checklist for evaluation of Cleaning / Retention aspects for the potential of cross-contamination.
-
- Identify the gaps through the checklist and improve the cleaning procedure to meet the criteria or use dedicated equipment.
-
- For Containment Approach (CA) 4 – Validated automatic cleaning cycle is recommended; swab cleaning verification is required for manual cleaning, applicable for main product equipment items.
-
- For Containment Approach (CA) 5 – Automatic cleaning is required, applicable for main product equipment items.
-
Evaluate the process for containment approach (Mechanical Transfer and Airborne transfer):
-
- Is the potential for mechanical transfer controlled to a safe predetermined level?
-
- Refer to Annexure 5 – Checklist for evaluation of Mechanical Transfer aspects for the potential of cross-contamination.
-
- Identify the gaps through the checklist and introduce the required controls to minimize the mechanical transfer.
-
- Is the potential for airborne transfer controlled to a safe predetermined level?
-
- Refer to Annexure 6 – Checklist for evaluation of Airborne Transfer aspects for the potential of cross-contamination.
-
- Identify the gaps through the checklist and introduce the required controls to minimize the airborne transfer.
Note: The risk level calculated through this matrix may be upgraded to a higher level, if needed, based on a case-to-case evaluation by QA.
-
- Based on the Risk Analysis results, the concerned site(s) shall perform an assessment of controls available to manufacture the product with proper containment.
-
- If controls at the site are sufficient to maintain containment to an acceptable level, the new products can be manufactured at the site.
-
- In case the controls are not available at the site, the following activities can be performed:
-
-
- Tentative acceptance with a list of the activities/modification required for acceptance.
-
-
-
- In special cases and If applicable, product-specific risk assessment, addressing the materials, the process, and controls, which will ensure that the potential risk is acceptable.
-
-
-
- A deliverable of these assessments may be a batch-based protocol, for implementing temporary controls that will allow the manufacture of the product at an acceptable risk level.
-
-
-
Change Control procedure shall be followed for new product manufacturing at the site as per the laid down procedures.
-
-
- Based on the type of separation category required, well-defined engineering controls and administrative GMP operating practices as defined by this document shall be in place so as to prevent contamination.
-
- Plant Management shall be responsible for providing required material, manpower, and other resources for establishing effective and GMP compliant product separation management practices.
-
- The plant shall be able to deal simultaneously with different stages of the manufacturing of several products under conditions that minimize the risk of cross-contamination of one product with another.
-
- Whenever possible, manufacturing operations should be designed to be carried out in closed systems (e.g. enclosures at dust-generating points) to prevent dust generation.
-
- The generation of dust will consequently be reduced at the source and therefore the risk of cross-contamination will be minimized.
-
- If this is not possible all equipment used to process granules/powders/products should be fitted with adequate means of dust extraction.
-
Separation Category Requirements For Ancillary Areas:
- Development Laboratories and QC Laboratories generally shall not require separation since products handled in the laboratory are not marketed, dispensed to clinical study sites, or exposed to products that are marketed or used in clinical study sites.
-
- Thus, multiple products (potent as well as non-potent) may be tested/developed in the same laboratory.
-
- However, all material and personnel in that laboratory shall be considered contaminated with the potent drug, if potent drugs pass through the laboratory.
-
- Additionally, man and material movement shall be organized in a manner that will prevent chances of cross-contamination.
-
- Development Laboratories where products are manufactured to be dispensed to clinical study sites or exposed to products that are marketed or used in clinical study sites use shall be reviewed for the separation category as discussed for manufacturing facilities.
-
- Secondary packaging and warehousing operations that occur after the final container has been filled and sealed similarly shall not require separation routinely, if there is no product exposure after sealing.
-
- Cafeterias shall also be considered as contaminated if personnel working in the manufacturing of potent drugs and/or laboratory area(s) are visiting the cafeterias.
-
- Hence, in order to guarantee personnel safety as well as prevent cross-contamination through personnel, all the personnel working in the manufacturing of potent drugs and/or laboratory area(s) shall have a previous decontamination procedure (e.g. for Gowning / Degowning, use of Air showers, etc.) for change room before accessing the areas such as cafeterias, etc.
-
Continuous Monitoring Of Separation Controls:
-
- Separation controls shall be routinely audited/reviewed; the procedures shall be validated and appropriately monitored to assure continued effectiveness (E.g. by analytical testing, data trending of environmental monitoring samples, etc.).
-
Requirements:
- Products shall be transferred to sites with a minimum of 3 months of stability data (Accelerated and Long Term) of 1 R&D batch.
-
- This stability data should be part of the Master Product Specification (MPS) given by R&D.
-
- Based on the above satisfactory stability data R&D shall give pre-approval to site to start manufacturing 1 optimization batch (Scale-up) and 3 commercial process validation batches.
-
- The Plant can manufacture 1 optimization batch (Scale-up) and 3 commercial process validation batches.
-
- All 3 commercial process validation batches should be charged on stability.
-
- Optimization batch should be considered as a commercial batch, only if there is no change in manufacturing formula and process compared to 3 process validation batches.
-
- In case during optimization batch there is a change, which can impact the stability of product like (but not limited to)
-
-
- Change in Batch Size,
-
-
-
- Change in the category of Process Equipment (working on a different principle of operation, such as tumbler blending to RMG, etc.),
-
-
-
- critical process steps and/or Critical process parameters or change in manufacturing formula and process then optimization batch should not be considered as a commercial batch.
-
-
- These commercial batches should be released or launched to market only after receipt of satisfactory 6-month stability data (Accelerated and Long Term) of 1 R&D batch and satisfactory minimum 3-month stability data(Accelerated and Long Term) of 3 commercial Process Validation batches with final approval from R&D.
-
- Further commercial batch stability up to the proposed shelf life should be continued at site and monitored.
7.0 ANNEXURES:
-
Annexure 1 – Specific Regulatory Requirements for Products to be handled in Dedicated Facilities.
| Sr. No. | Regulatory Agency | Drug Classes Which Requires Dedicated Facility |
| 1 | European Marketing Authorization (EMEA) | Certain antibiotics, certain hormones, certain Cytotoxics, certain highly active drugs, and nonmedical products. |
| 2 | Schedule M, Drugs and Cosmetics Act (India) | Biological Preparations (with live microorganisms), Beta-Lactam Antibiotics, Sex Hormones, and Cytotoxic substances. |
| 3 | The United States Food and Drug Administration (USFDA) | Beta-Lactam Antibiotics and Cephalosporins |
| 4 | Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operation Scheme(PIC/s) | Highly sensitizing materials (penicillin), biological preparations (live micro-organisms), certain antibiotics, certain hormones, certain cytotoxics, certain highly active drugs, and non-medical products |
Note:
|
||
-
Annexure 2 – The Separation Categories with Recommended Controls associated with each category.
| Dedicated Building / Facility. | ||
| Controls Required | 1 | A dedicated building/facility for the group of materials in order to eliminate the risk of cross-contamination to other products. |
| 2 | Cross-contamination controls of products within the facility should be in compliance with the hazard level of the materials. | |
Containment Approach 1 – Multi-Product Area: |
||
| Controls Required | 1 | Only authorized personnel should be allowed into the working area. |
| 2 | Training must be provided on decontamination prior to leaving the work area. | |
| 3 | Generally applicable to areas handling products in small quantities, with medium or low dustiness (i.e. laboratories/IPC test) which will not be commercialized. | |
| 4 | No hazardous material handling. | |
| 5 | No special engineering controls, with adequate control effected by
general ventilation and good manufacturing practice. |
|
| 6 | Use of validated facility and equipment, routine cleaning, and monitoring procedures. | |
Containment Approach 2 – Low-Risk Production Area: |
||
| Controls Required | Access | |
| 1 | Only authorized personnel should be allowed into the working area. | |
| 2 | Training must be provided on decontamination prior to leaving the work area. | |
| Process | ||
| 1 | Proper sequence scheduling of pharmaceutical production runs (e.g. campaign production). | |
| Air Movement Control | ||
| 1 | Qualified HVAC systems shall be available complying area classification requirements for the manufacturing process. | |
| Maintenance And Cleaning | ||
| 1 | Surface finishes must be easy to clean and non-porous. | |
| 2 | A regular maintenance and cleaning schedule for equipment and surfaces should be implemented. | |
| 3 | Cleaning shall be by vacuum or wet mopping. Dry brush sweeping and compressed air cleaning should be avoided. | |
| Training | ||
| 1 | Specific training is required on the hazardous nature of the substances handled and the operation of the controls. | |
Containment Approach 3 – General Production Area: |
||
| Controls Required | Access | |
| 1 | Entry to the working area should be controlled. | |
| 2 | Processing areas and accesses with appropriate pressure differentials | |
| 3 | De-gowning upon exit from the production area. | |
| Isolated Operator Control | ||
| 1 | Boundaries of the manufacturing area/suits shall be clearly defined. | |
| 2 | Only limited breaching of containment. E.g. the taking of samples is permitted. | |
| 3 | The room should be maintained under negative pressure relative to adjacent areas in order to prevent leakage. | |
| 4 | Contaminated air from the extraction system must be passed through a suitable safe change HEPA filter. | |
| 5 | Containment capability should be demonstrated by studies. | |
| Maintenance And Cleaning | ||
| 1 | Surface finishes should be crevice-free & ground smooth to effect easy cleaning. | |
| 2 | A regular maintenance and cleaning schedule for equipment and
surfaces should be implemented. |
|
| 3 | Equipment design should facilitate easy maintenance. | |
| Training | ||
| 1 | Specific on-the-job training is required. This should include an understanding of the plant. The maintenance and use of PPE and procedures to detect and deal with the loss of containment. | |
| 2 | Periodic retraining/refresher training will be required. | |
Containment Approach 4 – Process containment (recommended), or high containment production area: |
||
| Controls
Required |
Access | |
| 1 | Entry to the working area must be controlled. | |
| 2 | Processing areas and accesses with appropriate pressure differentials and buffer zones (i.e. Redundant pressure cascades). | |
| 3 | Restricted personnel and material movements. | |
| Only operators trained in emergency evacuation procedures will be allowed access to the area | ||
| Isolated Operator Control | ||
| 1 | Isolation of operators from the process via direct connections, or transfer, between the process vessels and containers. | |
| 2 | Enclosures should be maintained under negative pressure to prevent leakage. | |
| 3 | Contaminated air from the extraction system should be passed through a suitable safe change HEPA filter before exhausting outside the building. | |
| 4 | Dedicated HVAC System, redundant HEPA filtration or return air, or 100% fresh air. | |
| 5 | Regular certification and testing of the filtration system will be required. | |
| 6 | Containment capability should be demonstrated by studies | |
| Maintenance And Cleaning | ||
| 1 | Equipment design should facilitate easy maintenance. | |
| 2 | When applicable, new equipment should be compatible with automated cleanings such as WIP or CIP systems. | |
| 3 | A regular maintenance and cleaning schedule for equipment and the surface must be implemented. | |
| 4 | Equipment design must facilitate easy maintenance. | |
| 5 | Special procedures, such as purging or cleaning procedures such as CIP are recommended before systems are opened. | |
| Personnel Gown | ||
| 1 | Use of a disposable gown recommended. | |
Containment Approach 5 – Full process containment |
||
| Controls
Required |
Access | |
| 1 | Entry for only authorized personnel. | |
| Totally Isolated Process | ||
| 1 | (Robotics. etc.) These designs will typically be multiple layer
containment, which uses e.g. a totally sealed process with fully welded pipe connections. |
|
| 2 | Equipment design will be of a specialist design. | |
| 3 | The operator interface should be as minimal as possible. | |
| 4 | Containment capability should be demonstrated by studies. | |
| Maintenance And Cleaning | ||
| 1 | Automated decontamination/CIP of the process will be necessary prior to any opening of the process area. | |
| Personal Protective Equipment | ||
| 1 | Properly designed gowning procedures/Use of space suits/Use of Exit Showers/mystifiers | |
-
Annexure 3 – Checklist for Evaluation of Mix-up.
| Sr. No. | Check Point | Check | ||
| Yes | No | |||
|
1 |
Facility and Equipment Design/Controls |
|||
| 1.1 | Facility Design Aspects– is the facility layout such that there are no shared process flows or areas of process overlap? (Overlapping process flows and transit routes, common dispensary areas, common storage areas for change parts, common manual cleaning areas where dirty and clean equipment from different processes may be present at the same time, etc.) Are dedicated corridors/access provided where needed? | |||
| 1.2 | Is the Facility’s Spatial Configuration suitably designed – with Process Boundary, Room Boundary, and GxP Boundary wherever required? (Such boundaries may be provided by means of inter-lockable Air Locks/Barrier Walls, etc.) | |||
| 1.3 | Is the process flow design appropriate to keep clean and dirty equipment, people, materials, etc. separate? | |||
| 1.4 | Do all GxP areas/equipment have hard, washable, coved and crevice-free finishes that are sufficiently leaking tight to enable pressure cascades to be created and maintained? | |||
| 1.5 | Air Handling Philosophy: Dedicated HVAC Systems OR Shared HVAC systems with high-efficiency filters in the terminal supply/in the return ductwork/exhaust of ductwork or ‘Once Through’ type of HVAC System. | |||
| 1.6 | Dedicated Critical Utilities (e.g. Purified Water Generation System and Re-circulation Loop, Vacuum Line, WFI Loop, Compressed Air Line, etc.) | |||
| 1.7 | Dedicated Corridors/Access. | |||
| 1.8 | Dedicated process equipment/dedication of product contact parts/ dedication of selected parts which are harder to clean (e.g. filters)/ dedication of maintenance tools/use of disposable technologies. | |||
| 1.9 | Pressure Regimen in the Facility which is protective of product, personnel, and environment. | |||
| 1.10 | Containment Technologies in the Equipment /Facility. (E.g. Scrubbers, Isolators, Barriers, Localized extraction system for controlled removal of dust close to the source of contaminant, etc.) | |||
| 1.11 | Use of automatic Clean-In-Place (CIP) system in the equipment of validated effectiveness. | |||
| 1.12 | Identify areas where the existing controls may fail. Are additional controls needed? | |||
|
Sr. No. |
Check Point | Check | ||
| Yes |
No |
|||
|
2 |
Procedures/SOPs |
|||
| 2.1 | Is there a procedure for the status labeling of equipment and facilities? Or if not, can the procedures and controls effectively prevent cross-contamination? | |||
| 2.2 | Is there a procedure for physical segregation, process flow, and security during material and product receipt, handling, storage, and staging? | |||
| 2.3 | Is there a robust line clearance procedure in place? | |||
| 2.4 | Is there a procedure for maintaining dedicated laundry? (i.e. keeping protective clothing inside areas where products with a high risk of cross-contamination are processed) | |||
| 2.5 | Identify areas where the existing procedures may fail or the consequences if the procedures are not followed. | |||
| 2.6 | Are additional procedures needed? | |||
| 2.7 | Are the existing procedures too complicated to follow? | |||
| Remarks | ||||
-
Annexure 4 – Checklist for Evaluation of Cleaning / Retention Aspects.
| Sr. No. | Check Point | Check | ||
| Yes | No | |||
| 1 | Check whether an acceptable level of retention or carryover is determined, taking into account the hazard(s) presented by the product and the nature and route of administration of the product that will be processed next in the equipment, unit, or facility. | |||
| Check Feasibility of the Cleaning Criteria | ||||
| 1.1 | If a very low level of acceptable carryover is defined, are there analytical methods that will achieve the level of detection required? | |||
| 1.2 | If an aggressive cleaning agent (e.g., caustics or acids) is used, what effects will this cleaning agent have on the equipment or materials of construction? Will there be pitting or corrosion of surfaces with long term use? Will equipment parts become corroded or damaged over time (e.g., gasket materials)? | |||
| 1.3 | Check Practicability of the Cleaning Program | |||
| 1.4 | Would the effort required to perform a cleaning to the defined level be practical? | |||
| 1.5 | Would the facility need to be shut down for an extended period? | |||
| 1.6 | Would extra personnel be required to undertake the cleaning? | |||
| 1.7 | Would high volumes of solvent or other chemicals be required presenting a different risk scenario (e.g., exposure of workers to the cleaning materials, containment, and cost of disposal of solvents)? | |||
| Remarks | ||||
-
Annexure 5 – Checklist for Evaluation of Mechanical Transfer aspects.
|
Sr. No. |
Check Point | Check | ||
| Yes |
No |
|||
| 1 | Process | |||
| 1.1 | Is the process closed? Can material escape the process and be transported around the facility via equipment, product, intermediates, supplies, and personnel moving through the facility? (i.e. check whether process/room/GxP boundary is provided wherever needed). | |||
| 1.2 | Is there a system to change garments at the product boundary? | |||
| 1.3 | Is there a procedure for maintaining dedicated laundry (i.e. keeping protective clothing inside areas where products with a high risk of cross-contamination are processed). |
|
|
|
| 1.4 | Check surface-surface contact, e.g., scraping, smearing, or wiping of process equipment. | |||
| 1.5 | Check whether there is the repeated transfer of material to or from unit processes through breakable or poorly contained couplings. | |||
| 1.6 | The requirement of the mobility of equipment/personnel due to use in multiple areas. Lesser the mobility, the better the control to prevent cross-contamination. | |||
| 2 | Procedures | |||
| 2.1 | Are procedures and/or controls in place to minimize cross-contamination
risk by mechanical transfer due to garments? |
|||
| 2.2 | Are procedures and/or controls in place that states equipment is to be wiped down prior to leaving the processing rooms? | |||
| 2.3 | Are there means to prevent the transfer from feet or equipment/trolley wheels? | |||
| 2.4 | Is de-contamination required to be carried out? If yes, is there a robust decontamination procedure in place? | |||
| 2.5 | Is there a procedure in place for waste isolation and handling contaminated rinsing water and soiled gowning? | |||
| 2.6 | Is there a procedure for monitoring of working behavior to ensure training effectiveness and compliance with the relevant procedural controls? | |||
| 2.7 | Is there a procedure in place for the separation of contract service provider activities? | |||
| 2.8 | Is there a procedure for recording and handling of spills/accidental events which may cause cross-contamination? | |||
| Remark | ||||
-
Annexure 6 – Checklist for Evaluation of Airborne Transfer aspects (Example Template).
|
Sr. No. |
Check Point | Check | ||
| Yes |
No |
|||
| 1 | Process | |||
| 1.1 | Is the processing system a closed one or is the product exposed to the room environment during processing, transfer, or cleaning? Can material escape the process? | |||
| 1.2 | Are adequate engineering controls available to reduce the degree of a process’ openness? | |||
| 1.3 | Is the airflow pattern from ceiling to low level exhaust appropriate so that the operator and room entry is in the ‘clean zone’ and the process is in the ‘dirty zone’? | |||
| 1.4 | Is there an appropriate gowning regimen to minimize the risk of cross-contamination by airborne transfer? | |||
| 1.5 | Is there an appropriate decontamination procedure (if needed) to minimize the risk of cross-contamination by airborne transfer? | |||
| 1.6 | Check whether process materials transmissible as aerosols must have certain physical properties to maintain a stable aerosol for a significant time period. | |||
| 1.7 | Check for risks of poorly sealed process equipment with pressure driving forces to promote undesired airborne transfer, e.g., positive pressure at source and/or negative pressure at receiving material positions. | |||
| 2 | Facility | |||
| 2.1 | Are the facility design aspects appropriate/adequate to control airborne transfer? (Interlockable airlocks, HVAC system, pressure regimen, etc.) | |||
| 2.2 | Check whether the relative location of different process streams is adequate enough to minimize cross-contamination. | |||
| Remark | ||||
-
Annexure 7 – Example of Evaluation Form of Containment Approach.
| Product Name and Strength | Formulation Type | ||
| API Name | Batch Size | ||
| Drug/API Classification | {Pharmacological Class of Drug} | ||
| PDE/ADE Value | Hazard Category | ||
Evaluation for Dedicated Facility:
| Sr. No. | Check Point | Check | |
| Yes | No | ||
| 1 | Any specific regulatory requirement to handle the product in a dedicated facility based on Annex 1. | ||
| 2 | Scientific data from the toxicological evaluation support a controllable risk (e.g. allergenic potential from highly sensitizing materials such as beta-lactams). – I.e. PDE is available. | ||
| Remark | {Non-compliance to any checkpoint mentioned above require a dedicated facility for the manufacture of the product} | ||
| Sr.
No. |
Manufacturing
Stage |
Area
(Room Name & No.) |
Qty. of API handled | Exposure Potential | Time | Band | Hazard Category | Containment Approach | Controls |
| If the information available, put details. Otherwise, keep as NA |
|
Low
Medium High |
□ Short
□ Long |
1.Available
2. Procedural al controls required 3. Engineering activity require (equipment transfer, controls addition, etc.) |
- Conclusion: {It shall include the containment approach derived for each area and the evaluation of current controls of respective processing areas for the manufacturing of the new products. Based on this, a conclusion shall be drawn for acceptance of the product for manufacturing in facility/area OR up-gradation of current controls/Introduction of new controls required for manufacturing of the new product.}
| Require dedicated facility | Yes □ No □ |
| Accepted for manufacturing with current controls | Yes □ No □ |
| Required up-gradation of current controls/introduction of new controls. | Yes □ No □ |
(Select the appropriate option “√”)
List out the new controls, if required, for acceptance of manufacturing of new product:
| Item no. | Gap/ lack of Control | Proposed activity | Responsibility | Target Completion Date |
Corrective action Verification and approval:
| Item no. | Gap/ lack of Control | Proposed activity | Responsibility | Target Completion Date | Completion date |
-
Annexure 8 – WorkFlow Chart.
******************************************** END *****************************************


