 The Acceptable Quality Level (AQL) is a percent defective that is the baseline requirement for the quality of the producer’s product. The producer would like to design a sampling plan such that there is a high probability of accepting a lot that has a defect level less than or equal to the AQL.
The Acceptable Quality Level (AQL) is a percent defective that is the baseline requirement for the quality of the producer’s product. The producer would like to design a sampling plan such that there is a high probability of accepting a lot that has a defect level less than or equal to the AQL.
SOP for Acceptable Quality Level (AQL) Inspection
1.0 PURPOSE:
-
- To define the procedure for Acceptable Quality Level sampling for Tablets and Capsules during bulk approval.
2.0 SCOPE:
-
- This procedure is applicable to bulk approval of manufactured Tablets and Capsules at pharmaceutical product manufacturing locations.
3.0 REFERENCES:
-
- SOP for Event Reporting and Investigation.
4.0 Responsibilities:
-
-
Production Department:
-
-
- Intimate QA Officer for bulk inspection and to perform online Acceptable Quality Level (AQL) sampling.
-
- Ensure simultaneous entries in the sequential log of respective equipment/area if applicable.
-
- Ensure the handling and storage of the drug product as per BMR.
-
- Perform 100% inspection as and when acceptable Quality Level (AQL) is failed.
-
- Investigate in case the event is raised for Acceptable Quality Level (AQL) inspection failure.
-
-
Quality Assurance (QA) Department:
-
-
- Prepare and implement the SOP .
-
- Perform the Acceptable Quality Level (AQL) sampling and inspection as per SOP.
-
- Ensure correct and complete documentation in BMR/applicable annexure.
-
- Sample and inspect the bulk product to establish if the product meets an acceptable quality level.
-
- Ensure that an event is raised if Acceptable Quality Level (AQL) inspection fail (as per criteria defined in this SOP) and the same shall be addressed in the batch record.
-
-
Regulatory Affairs, Quality Head and Plant Head:
-
-
- Review and approve the investigation reports in case if a batch does not meet Acceptable Quality Level (AQL) as per the criteria defined in this SOP.
-
- Review and approve the SOP.
5.0 ABBREVIATIONS:
-
- ANSI: American National Standards Institute
-
- AQL: Acceptable Quality Level
-
- ASQ: American Society for Quality
-
- BMR: Batch Manufacturing Record
-
- CAPA: Corrective and Preventive Action
-
- ERP: Enterprise Resources Planning
-
- NA: Not Applicable
-
- QA : Quality Assurance
-
- QC: Quality Control
-
- SFG: Semi Finished Goods
-
- SOP: Standard Operating Procedure
6.0 DEFINITIONS OF TERMS:
-
-
Inspection by attributes:
- Inspection whereby either the unit of product is classified simply as defective or no-defective or the number of defects in the unit of product is counted, with respect to a given requirement or set of requirements.
-
-
-
Acceptable Quality Level (AQL):
- The AQL is a percent defective that is the baseline requirement for the quality of the producer’s product. The producer would like to design a sampling plan such that there is a high probability of accepting a lot that has a defect level less than or equal to the Acceptable Quality Level (AQL).
-
-
-
Sampling Plan:
- A lot sampling plan is a statement of the sample size or sizes to be used and the associated acceptance and rejection numbers.
-
-
-
Representative Sampling:
- When appropriate, the number of units in the sample shall be selected in proportion to the size of sub-lots or sub-batches, or parts or the lot or batch, identified by some rationale criterion. When representative sampling is used, the units from each part of the lot or batch shall be selected at random.
-
-
-
Defects:
- A defect is any non- conformance of the unit of the product with the specified requirement.
-
-
-
Critical Defect:
- A critical defect is one which is likely to result in a hazardous or unsafe condition for individual using, maintaining or depending upon that product.
-
-
-
Major Defect:
- A major defect is one, other than critical, that is likely to result in failure or to reduce materially and usability of the unit of product for its intended purpose..
-
-
-
Minor Defect:
- A Minor defect is one that is not likely to reduce considerably the usability of the unit of product for its intended purpose or is a departure from established standards having little bearing on the effective use or operation of the unit of product.
-
-
-
Part Lot:
- Distinct portions of a whole lot, i.e. a whole lot of core tablets divided into an equal portion for the purpose of coating – each portion of the coated tablets is a distinct lot. I
-
-
-
Inspection Levels:
- The standards provide for three general inspection levels (i.e. Level I-Reduced Inspection, Level II- Normal/General Inspection, Level III- Tightened Inspection) and four special inspection levels. These levels permit the user to balance the cost of inspection against the amount of protection required.
-
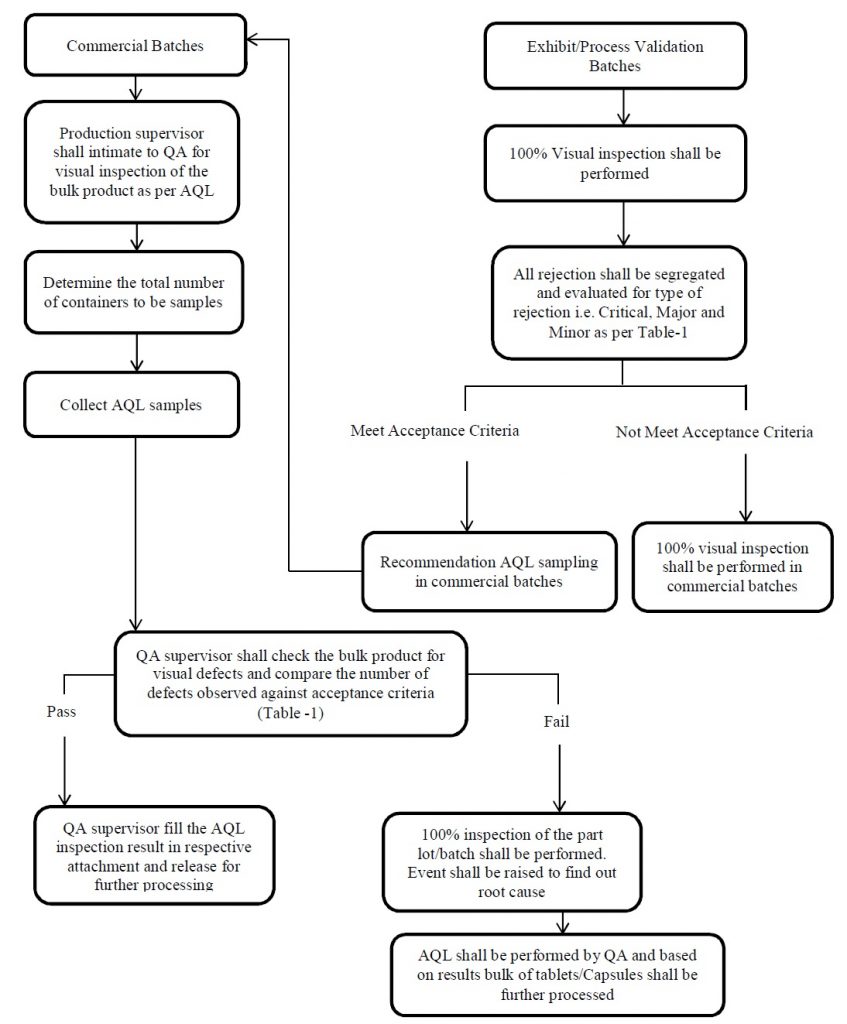
7.0 PROCEDURE FOR ACCEPTABLE QUALITY LEVEL (AQL):
-
- Acceptable Quality Level (AQL) checks shall be performed semi-finished for commercial batches of tablets and capsules.
-
- QA shall perform the Acceptable Quality Level (AQL) checks in the respective area and based on findings,
-
- QA shall decide the need & extent of inspection for the subjected batch and details shall be recorded.
-
- In the case of Process validation batches, 100% visual inspection shall be performed and the same shall be addressed in the Batch record.
-
- During Process validation, Segregate the visual inspection rejection and evaluate the type of rejection i.e. Critical, Major and Minor.
-
- If the visual inspection trend of process validation is satisfactory, then based on process validation (report) recommendations, Acceptable Quality Level (AQL) sampling shall be performed in commercial batches.
-
-
Selection of Containers for Acceptable Quality Level (AQL) Sampling of Tablets/Capsules:
-
-
- Production Officer shall submit duly filled and signed BMR to QA for review and intimate for visual inspection of the bulk product as per Acceptable Quality Level (AQL).
-
- QA shall ensure Product name, Batch No, Manufacturing date, Expiry date and select the containers of product for Acceptable Quality Level (AQL).
-
-
The samples to be withdrawn for the bulk approval from the number of the container shall be based on the following criteria :
-
-
- Determine the “total number of containers to be sampled” per part lot (for coated tablets) or per batch (for capsules and uncoated tablets) by using the formula 10+√n +1, Where “n” is the total number of containers per part lot/Batch.
-
- If the total number of the container is less than or equal to 10 Nos (per batch/lot), then samples shall be checked from all the containers.
-
- In case of the total number of the container is more than 10, then for Acceptable Quality Level (AQL) sampling of 10 containers shall be done 100% and the remaining container shall be AQL as per formula √n+1.
-
-
For Example Number of the container is 35 then AQL of 10 (Frist 5 + Last 5) container is 100% and for remaining (35-10=25) 5 containers, as per formula (√25+1=5+1), 6 containers shall be checked.
-
-
- If any value of √n is above the whole number, the number shall be rounded off to the next whole number).
-
- For coated tablets, if coating performed in multiple lots then individual lot size shall be considered as batch size and accordingly Acceptable Quality Level (AQL) samples shall be withdrawn.
-
- E.g. If 1 part lot contains 28 containers ( e.g. coating is performed in two lots) then the number of containers to be sampled shall be 10+√18+1 = 10+4.24+1 =15.24 Therefore the total number of containers to be sampled shall be 16 from each lot.
-
- If one batch contains 50 containers (e.g. uncoated tablets/capsules) then the number of containers to be sampled will be 10+√40+1 = 10+6.32+1 = 17.32, therefore, the total number of containers to be sampled shall be 18 from the whole batch.
-
-
Collection of samples and acceptance criteria for Acceptable Quality Level (AQL)
-
-
- Collect the samples from each selected container in equal quantity in a duly labeled polyethylene bag.
-
- Check each collected tablet (coated and uncoated) or capsule for the quality attributes specified as per Acceptable Quality Level (AQL) on the inspection trolley.
-
- e.g. In case of uncoated tablets/ capsules if Acceptable Quality Level (AQL) sample quantity requirement is 1250 and no. of containers to be sampled is 9 then from each container 1250/9 i.e. 138.9=139 samples (Withdrawn of samples = No. of Sample X Avg. weight of sample or by Manual Counting) shall be withdrawn and composite sample of 1250 bulk units shall be prepared.
-
- In case of coated tablets if the coating is performed in two lots and lot size is 500000 then 800 tablets from each part lot shall be withdrawn.
-
- If samples are to be withdrawn from 5 containers then from each container 800/5=160 coated tablets (Withdrawn of samples = No. of Sample X Avg. weight of sample or by Manual Counting) shall be withdrawn.
-
- For the purpose of the Acceptable Quality Level (AQL). Consider the sampling standard weight of tablets as average weight.
-
- QA Officer shall check the bulk product for visual defects as per the below-mentioned procedure. ( Check the visual defects on both the sides of tablets in case of tablet products ).
-
- Collect the sample quantity from each selected container in equal quantity and inspect on the inspection trolley.
-
-
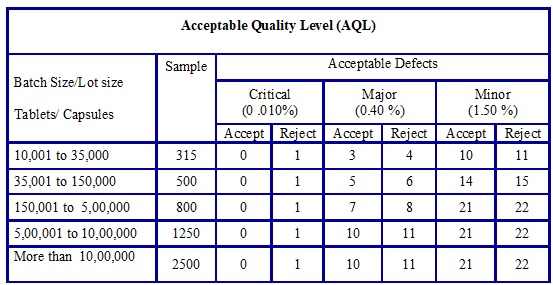
The acceptance criteria for Acceptable Quality Level (AQL) shall be as given below based on the classification of the defect.
-
| Sr. No. | Classification of defect | Acceptance criteria |
| 01 | Critical | 0.010% |
| 02 | Major | 0.40 % |
| 03 | Minor | 1.50 % |
-
- The sampling quantity for bulk approval and number of defects observed against acceptance criteria to determine whether batch passes or fails shall be as per the below table.
-
-
The below table is equivalent to the General Inspection Levels -LEVEL II.
-
Table-1
-
- In case batch size is more than 10,00,000 Tablets/Capsules sample size quantity is doubled as compared to if batch size is between 5,00,001 to 10,00,000 Tablets/ Capsules.
-
- This is in-house stringent criteria with respect to sample quantity in order to increase sample size in proportion to batch size.
-
- Note: If batch/part lot fails in Acceptable Quality Level (AQL) acceptance criteria then 100 % inspection of the part lot/batch shall be performed. Raise the event to find out the root cause.
-
- The classification of defects as per the nature of dosage forms as given below:
-
-
Classification of core tablets defects are as follows:
-
(1) Critical |
|
| A. Wrong appearance | |
| Foreign product | Pharmaceutical material is either a component, powder on the finished dosage that is not a normal part of the batch being processed |
| Foreign material | Anything other than Pharmaceutical material that is not a normal part of the batch being processed. |
| Wrong appearance | Product appearance is not as per product specification. |
| Tablets not in uniform size /Wrong punch shape | The tablet that is notably thinner, thicker, larger, smaller or a different shape than the other in the sample. |
| Abnormal discoloration of products | Discolored tablets |
|
Note: Broken tablets are considered as critical criteria and 100% inspection shall be performed prior to loading bulk units into the hopper for primary packaging. |
|
(2) Major : |
|
| Chipping or Minor breaking | It is the breaking of tablet edges, while the tablet leaves the press or during subsequent handling and coating operations. |
| Illegible de-bossing | Characters in the de-bossing are not legible. |
| Illegible embossing | Characters in the embossing are not legible. |
| Layer separation | In the bilayer tablet, one layer is separated from the other layers. |
| Lamination | It is the separation of a tablet into two or more distinct horizontal layers. |
| Cracking/broken tablet | Small, fine cracks observed on the upper and lower central surface of tablets, or very rarely on the sidewall are referred to as ‘Cracks’. |
| Double impression | ‘Double Impression’ involves only those punches, which have a monogram or other engraving on them. |
| Soft tablets | The tablets are susceptible to hydrolysis will develop soft nature. |
| Picking | The small amount of material from a tablet is sticking to and being removed off from the tablet-surface by a punch face. |
| Sticking | The tablet material adhering to the die wall. Filming is a slow form of sticking and is largely due to excess moisture in the granulation |
| Dark Spot/Blackspot/Colour particles | Stains or spots will appear on the tablet surface.
Migration of coloring agent upon storage. |
| Capping | Capping happened when the upper or lower segment of the tablet separates horizontally, either partially or completely from the main body of a tablet and comes off like a cap, during ejection from the tablet press, or during subsequent handling. |
| Binding | Tablets adhere, seize or tear in the die. A film is formed in the die and ejection of the tablet is hindered.
With excessive binding, the tablet sides are cracked and it may crumble apart |
(3) Minor : |
|
| Mottling | ‘Mottling’ is the term used to describe an unequal distribution of color on a tablet, with light or dark spots standing out in an otherwise uniform surface. |
| Rough surface | The tablet surface is rough. |
| Shade variation | The tablet that is visibly a different shade or color than the others in the sample. |
| Dust on Tablet | The powder found on the tablet. |
| De-bossing or score is not well defined | Characters in the de-bossing / crease have slight imperfections but are legible. |
-
-
Classification of Coated tablets defects are as follows:
-
(1) Critical : |
|
| A. Wrong appearance | |
| Foreign product | Pharmaceutical material is either a component, powder on the finished dosage that is not a normal part of the batch being processed. |
| Foreign material | Anything other than Pharmaceutical material that is not the normal part of the batch being processed. |
| Tablet not in uniform size | The tablet that is notably thinner, thicker, larger, smaller or a different shape than the other in the sample. |
| Missing debossing | Debossed characters are missing from the tablet. |
| Abnormal discoloration of products | Discolored tablets |
| Large dark staining on product | Stains found on the products |
|
Note: Broken tablet is considered as critical criteria and 100% inspection shall perform prior to loading bulk units into the hopper for primary packaging. |
|
(2) Major : |
|
| Blooming | It is a defect where the coating becomes dull immediately or after prolonged storage at high temperatures. |
| Bridging | This occurs when the coating fills in the lettering or logo on the tablet and is typically caused by improper application of the solution, poor design of the tablet embossing, high coating viscosity, a high percentage of solids in the solution, or improper atomization pressure. |
| Chipping | It is a defect where the film becomes chipped and dented, usually at the edges of the tablet. |
| Colour Variation | A defect which involves variation in the colour of the film. |
| Cratering | It is a defect of film coating whereby volcanic-like craters appears exposing the tablet surface. |
| Flaking | Film flakes off exposing the tablet surface |
| Mottling | ‘Mottling’ is the term used to describe an unequal distribution of color on a tablet, with light or dark spots standing out in an otherwise uniform surface. |
| Orange Peel/Roughness | It is a surface defect resulting in the film being rough and nonglossy. Appearance is similar to that of an orange |
| Splitting | The film splits usually around the edges of the tablet |
| Sticking | An indentation in the surface of the tablet that can cause a dimple resulting in weight variation. |
| Twinning | When two tablets stick tighter. It usually happens with capsule-shaped tablets. |
(3) Minor : |
|
| Blistering | The film becomes detached from substrate forming a blister |
| Blushing | It is a defect best described as whitish specks or haziness in the film. |
| Cracking/Splitting | It is a defect in which the film either cracks across the crown of the tablet (cracking) or splits around the edges of the tablet (Splitting) |
| Peel off | The film peels off exposing the best tablet surface |
| Picking | It is a defect where isolated areas of the film are pulled away from the surface when the tablet sticks together and then part. |
| Pitting | It is a defect whereby pits occur in the surface of a tablet core without any visible disruption of the film coating |
| Shade variation | The tablet that is visibly a different shade or color than the
others in the sample. |
| Infilling | It is a defect that renders the integrations indistinct. |
-
-
Classification of Capsules defects are as follows:
-
(1) Critical : |
|
| A. Wrong appearance | |
| Foreign Product | Pharmaceutical material is either a component, powder on the finished dosage that is not a normal part of the batch being processed. |
| Foreign Material | Anything other than Pharmaceutical material that is not a normal part of the batch being processed. |
| Missing Imprint On Cap & Body | All imprint characters are missing from the cap & body of the capsule that precludes product identification.
Wrong imprint. |
| Capsule Empty | Capsules with little or no contents or the body & cap are disengaged. |
| Partially filled capsule | Capsule not properly filled. |
(2) Major : |
|
| Capsule not free of cracks, breaks, notching, V notch cap, pinholes or splits. | The surface of the capsule is not intact & the contents of the capsule may fall out or have already fallen out. |
| Capsules not free of embedded surface spots. | Clearly defined particles embedded in the surface or the capsule. |
| Capsule not properly closed | Capsule not completely closed & the cap may slip off of the body. |
| Crushed Capsules | Translucent capsules with a crimped or smashed top.
Content leaking/ missing. |
| Imprint illegible | Characters in the imprint are not legible. |
(3) Minor : |
|
| Capsule not free of dents | Indentation in the surface of the capsule. |
| Capsule not free of surface blemishes | Clearly defined bumps, porous areas or lighter color areas prone to breakage. |
| Capsule cap & body cutting into one another (Telescoping) | A portion of the cap & body cutting the other, without loss of contents. |
| Blurred imprint | Characters in the imprint have a slight imperfection, including ink splatters, but are legible. |
| Inkspots | Two or more ink spots on the capsule away from the imprint. |
| Double Cap | The additional cap also observed on the body |
Note: Refer annexure-5 for flow chart of Acceptable Quality Level (AQL) procedure.
-
- If the bulk is meeting the acceptance criteria, production shall precede for weighing of the bulk, shall record batch reconciliation and yield data in the BMR and release the bulk for further processing.
-
- After completion of Acceptable Quality Level (AQL) sampling, the double polyethylene bag of sampled containers selected for AQL shall tie with fastener immediately.
-
- If the bulk is not meeting the acceptance criteria. QA shall ask the production Officer for visual inspection and raise the event to investigate the Acceptable Quality Level (AQL) failure.
-
- After visual inspection, again Acceptable Quality Level (AQL) shall be performed. Based on AQL results, Further proceed the bulk.
-
- If 3 consecutive batches fail in Acceptable Quality Level (AQL). Perform the investigation and Stop the subsequent manufacturing of drug products.
-
- The Acceptable Quality Level (AQL) inspection shall be performed in inspection cubical and if the Acceptable Quality Level (AQL) meets as per acceptance criteria then after the completion of AQL inspection inspected good Tablets / Capsules shall add to the last container of good Tablets / Capsules.
-
- 100% Inspection (By production department) and Acceptable Quality Level (AQL) inspection (By QA department) shall perform in the same condition i.e. area and inspection trolley etc.
-
-
In the case of an event that may impact visual inspection, a 100% inspection shall be performed.
-
-
- In case of repetitive market complaints related to visual inspection defects based on investigation findings AQL shall be discontinued and 100% visual inspection shall be performed.
-
- After completion of Acceptable Quality Level (AQL) inspection, QA Officer shall fill the AQL inspection result in annexure-1 in case of core Tablet, annexure-2 in case of Coated Tablet, annexure-3 in case of Capsules, and handover BMR to Production Officer for final yield and accountability reconciliation.
-
- QA shall send the finished samples of the core, Coated Tablet, and Capsules along with test requisition cum report to QC after AQL inspection.
-
- Production Officer shall submit duly filled and signed BMR to QA for the final release.
-
- QA shall check final yield, test requisition cum report and sign in reviewed by column in BMR and release the bulk in ERP as per location SOP.
-
- During the visual inspection in case of any abnormality observed investigation shall be triggered (like higher % of rejection for specific defect………etc.) to find out the root cause and initiate appropriate corrective and preventive action.
-
- Issue the AQL inspections annexure along with each BMR.
-
- Concerned Production Officer shall prepare the Acceptable Quality Level (AQL) Product list and QA shall check it as per Annexure-5.
-
- Perform AQL only for those products mentioned in the AQL List and remaining products which have problems as per their trend shall not be considered under AQL Product List and 100% visual inspection shall be performed for those products.
-
- In case Acceptable Quality Level (AQL) inspection is discontinued and 100% inspection started due to any failure then only upon implementation of CAPA. Start the AQL inspection in the particular drug product.
8.0 DISTRIBUTION OF ACCEPTABLE QUALITY LEVEL (AQL) SOP:
-
- Quality Assurance
-
- Production
9.0 ANNEXURES OF ACCEPTABLE QUALITY LEVEL (AQL) SOP:
Bulk release format for core Tablets (Annexure 1). Click here
AQL bulk release format for Coated Tablets (Annexure 2).Click here
AQL bulk release format for Capsules (Annexure 3).Click here
Bulk Approval Procedure of Acceptable Quality Level (AQL) for Tablets & Capsules (Annexure 4).
Acceptable Quality Level (AQL) Product List (Annexure 5). Click here



Pingback: Sampling of Packaging Materials - Procedure - Pharma Beginners