Standard Operating Procedure (SOP) for the handling of Pharmacopoeial Change at Drug Product Manufacturing Location. Handling when Pharmacopoeial changes in monographs and appearance of the new monographs for drug substances, excipients, drug products and updation or addition or deletion of general chapters.
Handling of Pharmacopoeial Change at Drug Manufacturing Location
1.0 Purpose:
-
- To define the systematic procedure for handling of pharmacopoeial change compliance of the impacted products manufactured drug Product Manufacturing Location.
2.0 Scope – SOP for Handling Pharmacopoeial Change:
-
- This procedure is applicable to the changes in monographs and appearance of the new monograph for drug substances, excipients, drug products and updation or addition or deletion of general chapters that are to be handled when appearing in the official pharmacopeia like USP, Ph. Eur., BP, IP, JP, etc. and in the pharmacopoeial forums.
-
- This guideline is also applicable for the monograph which shall be revised through revision bulletin or supplements.
-
- This guideline is also applicable for handling “Errata” appearing in various Pharmacopoeia.
3.0 References and Annexures :
-
- References :
-
- Transfer of Analytical Procedure – USP General Chapter <1224>
-
- Annexures :
-
- Guideline for applicability of pharmacopoeial changes as per market requirement. (Annexure-1)
-
- Format for register for handling pharmacopoeial changes maintained at the location. (Annexure-2)
-
- Format for the pharmacopoeial change evaluation report. (Annexure-3)
- Checklist for evaluation of impact analysis of Pharmacopoeial changes. (Annexure-4)
-
- Pharmacopoeial change Handling Flowchart. (Annexure-5)
-
- Individual product impact assessment cum closing report. (Annexure-6)
-
- Impacted Master List. (Annexure-7)
4.0 Responsibilities – SOP for Handling Pharmacopoeial Change:
-
Quality Assurance Head or Designee :
-
- Check for the applicability of pharmacopoeial changes and raise the allocation number for the application.
-
- Raise a change control for compliance of pharmacopoeial changes.
-
- Coordinate with QC for chemical evaluation.
-
- To coordinate with FDD for revised draft labels, revised MPS (if required).
-
- Coordinate with PDD for the preparation of artwork.
-
- To coordinate with ADD for revision of SPEC./STP and/or resolving analytical issues if any.
-
- To coordinate with Regulatory Affairs (if required) to obtain the product license.
-
- Coordinate with RMS to indent appropriate raw material.
-
- To coordinate with PMS to indent appropriate packaging material.
-
- Ensure the implementation of SOP.
-
- Coordinate with purchase for updated documents and material receipt as per pharmacopoeial status from the vendor for drug substances and excipients.
-
- To communicate as per the requirement with ADD/FDD/CQ in case of any non-compliance to pharmacopoeial change.
-
The analyst shall be responsible for :
-
- Analyze the sample as per the monograph.
-
- Prepare the evaluation report.
-
- Carry out an evaluation of impact analysis as per the market requirement for pharmacopoeial change and get confirmation from the Regulatory Affairs department.
-
- Revise the STP as per the revision of ADD STP.
-
Quality Control -Head or designee shall be responsible for :
-
- Plan for the evaluation of all impacted drug products/drug substances/excipients.
-
- Review the evaluation report.
-
- Intimate to ADD/FDD/CQ in case of any constraints regarding the evaluation of pharmacopoeial change.
-
- Inform QA Head or designee about the evaluation reports.
-
Analytical development head or designee shall be responsible for :
-
- Intimate the upcoming changes in pharmacopeia to Plant /Organic/FDD/ Purchase/ CQ/RA/Third party coordinator.
-
- Communicate with the pharmacopoeial agency for queries.
-
- Provide a justification report if the pharmacopoeial change is not adopted.
-
- Communicate with vendors in case of outsourcing API.
-
- Conduct analytical trials, if required.
-
- Circulate Errata to Plant /Organic/FDD/ CQ/RA/Third party coordinator.
-
Formulation Development Department/Organic synthesis department :
-
- Revise the manufacturing process/formulation and related documents i.e. MF/MPS/MFC as and when required to meet the requirement.
-
- Provide guidance for pharmacopoeial impurity nomenclature/structure and their identification against In house impurities.
-
- Intimate to PDD for updation of art-work, if necessary.
-
Packaging development Department :
-
- To update art work as instructed by FDD.
-
Corporate Quality Designee :
-
- Allocate the pharmacopoeial change numbers for a list of impacted material provided by the Quality control-head location.
-
- Review the pharmacopoeial changes evaluation report.
-
- Monitor the pharmacopoeial changes implementation status at manufacturing plants and maintain the status at CQ.
-
-
- To provide the status (Open) against allocated pharmacopoeial change number for materials impacted under the pharmacopoeial change to MDO as and when required.
-
-
Purchase Department :
-
- Coordinate with the Quality control-head of formulation plants, ADD and provide updated documents from vendors for drug substances and excipients.
-
- Ensure that the vendor provides materials as per updated STP.
5.0 Abbreviations – SOP for Handling of Pharmacopoeial Change:
-
- ADD Analytical development department.
-
- ANDA: Abbreviated new drug application
-
- BMR: Batch manufacturing record.
-
- BP: British pharmacopoeia.
-
- BPR: Batch packaging record. (SOP for BMR/BPR Review)
-
- CC: Change control
-
- COA: Certificate of analysis
-
- CQ: Corporate Quality.
-
- ERP: Enterprise resource planning
-
- EU: European Union
-
- FDD: Formulation development department.
-
- IH: In house
-
- IP : Indian pharmacopoeia.
-
- JP : Japanese pharmacopoeia.
-
- MDO: Managing director’s office.
-
- MF: Manufacturing formula.
-
- MPS: Master product specification.
-
- NA: Not applicable
-
- PDD: Packaging development department.
-
- PMS: Packaging Material Store
-
- Ph Eur: European pharmacopeia.
-
- RA: Regulatory affairs.
-
- RM: Raw material
-
- RMS: Raw Material Store
-
- ROW: Rest of world
-
- SLP: Standard Laboratory process
-
- SOP: Standard operating procedure
-
- Spec: Specification
-
- STP: Standard Testing Procedure
-
- USP: United states pharmacopeia
6.0 Procedure for Handling of Pharmacopoeial Change:
-
General instructions:
-
- The Head QA shall send the data of pharmacopoeial changes status to MDO as and when required.
-
- The Pharmacopoeial changed monograph should be made effective as suggested in individual monographs or within 6 months of a monograph published.
-
- If not made effective then the plant shall intimate the concerned reason to corporate quality for further action.
-
- If the monograph of drug substance/drug product is omitted/deleted from the pharmacopeia, then evaluate the impact on the revision of documents, packaging materials, and FDA licenses.
-
Handling of changes/appearance of Pharmacopoeial monograph of Drug Product:
-
- On receipt of pharmacopoeial supplement or the new version of pharmacopeia or errata, ADD shall intimate regarding the upcoming changes to plant /organic synthesis dept./FDD/ purchase/ CQ/RA/third party coordinator.
-
- QA shall make an entry in the applicable pharmacopoeial change intimation register.
-
- QA shall prepare the list of impacted monographs by referring to the product list provided by ADD.
-
- The list of products that are impacted shall be intimated by QA Head or designee to the CQ designee.
-
- On receipt of the impacted list from location, CQ designee shall allocate unique pharmacopoeial change numbers for all impacted cases and the same shall be intimated to location also.
-
- QA Head or designee shall raise a common change control to handle all the changes.
-
- Simultaneously entry of all the impacted products shall be done in Annexure-2 (Pharmacopoeial change handling register).
-
- For each impacted FP/API, Annexure-6 (Individual product assessment cum closing report) shall be issued and circulated to all concern for their consent on compliance.
-
-
All these issued and filled Annexure-6 shall be attached in the individual compliance file of each impacted FP/API.
-
-
- Photocopy of the filled Annexure-6 to be submitted with the relevant change control for the closure of the same.
-
- QA Head or designee shall prepare an impacted master list as per Annexure-7.
-
- However, other customized lists viz. impacted API List, impacted the Artwork list, impacted the license list, impacted the Spec.-STP list, impacted finished product list, impacted vendor list can also be prepared and relevant activities can be tracked from these customized lists.
-
- Even if there is no impact against any pharmacopoeial revision still QA Head or designee shall intimate to CQ designee for the respective pharmacopoeial changes stating “ No impact.”
-
- In the below-mentioned Table – 1, impact on various product aspects due to pharmacopoeial changes has been depicted in a chronological manner.
TABLE – 1
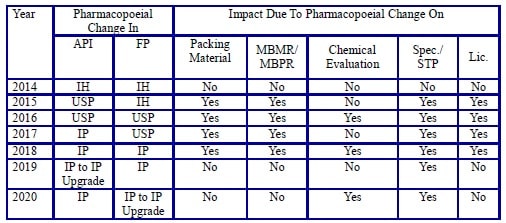
-
Pharmacopoeial Change Evaluation :
- After completion of location numbering of the impacted products, QA Head or designee shall plan for further course of action e.g chemical evaluation, revision of SPEC./STP, artwork revision, revision of product license, revision of masterbatch records, etc.
-
-
These activities can be initiated parallelly or can be prioritized as per requirement.
-
-
- QA Head or designee shall provide QC Head the list of drug products/drug substances whose evaluation is to be done.
-
- QC Head shall instruct the analyst to analyze one available batch and prepare evaluation report as per Annexure-3 and send a copy to ADD/CQ/QA for finalization of proposed specification and standard test procedure.
-
- Note: In case of multiple strengths, the evaluation shall be performed based on the trend analysis which may be the highest strength and addition to that any other.
-
- Preferably stability samples with maximum available impurity level and near to expiry.
-
- Control samples with maximum age shall be selected. API (maximum age sample) can be evaluated if required for the investigation.
-
- During the evaluation of the pharmacopoeial change method for related substances test, in-house impurities (which are monitored under in-house monograph) shall be injected and its system suitability parameters (resolution, retention time, etc) shall be verified and established.
-
- If during analysis, any analytical problem occurs, QC Head shall request ADD for support and send samples, if required for further evaluation.
-
- Note: If any obvious analytical error is identified during the review, then the test to be repeated.
-
- If the Analytical problem is confirmed at ADD, it shall be resolved by ADD or to be communicated to the pharmacopoeial agency, if required.
-
- Based on the response from the pharmacopoeial agency, if the monograph is not adopted, ADD shall prepare and provide a copy of the justification report to plant.
- Till the further solution, the existing monograph shall be used for analysis. (Petition to USP/ pharmacopoeial agency for the inclusion of Inhouse specification and methods shall be filed by ADD).
-
Following actions to be initiated regarding various kind of Chemical evaluation related issues:
-
- Analytical noncompliance:
-
- If the product does not comply with the pharmacopoeial requirement, the plant shall inform to ADD/FDD/Organic synthesis dept./CQ/RA for necessary actions.
-
- CQ designee shall make entry under the remarks column in the pharmacopoeial change register and monitor till the resolution of the problem.
-
- Formulation noncompliance:
-
- If it is confirmed that product noncompliance is due to formulation issues, then FDD/Organic synthesis department shall revise the formulation and consequently shall provide the revised SLP/MFC/MPS ect. to location.
-
- The location shall manufacture the batch as per revised MPS and QC shall evaluate it for pharmacopoeial compliance.
-
- Analytical & Formulation Compliance:
-
- If the product passes, ADD shall revise the STP as per the pharmacopoeial monograph and provide it to plant or plant shall revise the STP and Specification.
-
- It shall be done as per mutual agreement between plant and ADD.
Note: For adoption of the pharmacopoeial change method, method verification needs to be done by the plant.
-
- QC Head or designee shall carry out the evaluation of impact as per pharmacopoeial change (Annexure-3), through respective change control procedure.
-
- FDD/Organic synthesis shall revise the draft label and inform PDD to revise the artwork.
-
- PDD shall circulate revised artwork to location.
-
- FDD/Organic synthesis shall provide revised draft labels to plant QA for the revision of product licenses.
-
- Location QA shall obtain/update the product license from the state FDA.
-
- The location shall intimate Purchase about the desired grade of material. Purchase in turn shall communicate and ensure with the vendor that material shall be procured as per pharmacopoeial change.
-
- After receipt of the above documents location shall update the BMR, BPR, and other impacted documents.
-
- The location shall make the pharmacopoeial change effective only after ensuring completeness of all impacted aspects and the same shall be documented in Annexure-6.
-
- The plant shall start the analysis of drug product /raw material as per approved STP updated as per pharmacopoeial change.
-
- QA Head or designee shall inform the Analysis completed on, STP code/date, Artwork updated on, and FDA license received on to CQ designee against the allocated pharmacopoeial change number.
Note: Final closure date of the allocated change control number shall also be entered in the register (Annexure-2).
-
- CQ designee shall update the entries in the register for handling pharmacopoeial change (Annexure-2) and close the status.
-
- CQ designee shall inform the other location that the status of the respective allocated pharmacopoeial change number is closed and STP can be collected from the first plant.
-
Handling of changes/appearance of the Pharmacopoeial monograph of Drug Substances :
-
Drug substances (Outsource vendor -Domestic/ROW products):
-
-
- The plant shall intimate to Purchase.
-
- After getting mail confirmation/COA/STP as per pharmacopoeial change from the vendor, the purchase shall inform
-
- The plant shall revise the STP/Specification as per new pharmacopeia and shall make it effective as per the pharmacopoeial requirement.
-
- If the vendor is not providing the required pharmacopoeial grade e.g IP grade material, then location shall evaluate the material as per new monograph.
-
- When the material is passing as per new monograph, then code to code transfer of the material shall be done and the plant shall revise the STP/Specification as per new pharmacopeia and shall make it effective as per the pharmacopoeial requirement.
-
- If the material fails in analysis, then purchase shall be intimated regarding the failure results and an alternate vendor shall be managed to supply the required grade of material.
-
Handling of changes/appearance of the monograph of Excipients :
- Quality control-head or designee shall communicate with purchase to ensure that COA of material which complies the pharmacopoeial change is provided from the vendor.
-
- In the case of chromatographic method addition/changes, one batch (maximum age) shall be evaluated, if required for the suitability.
-
- If during analysis, any analytical problem occurs, the Quality control-head or designee shall request ADD or vendor for support and send samples if required.
-
- If the Analytical problem is confirmed by ADD or Vendor, it shall be resolved by ADD/Vendor or to be communicated to the pharmacopoeial agency, if required.
-
- Based on the response from the pharmacopoeial agency, if the monograph is not adopted, ADD shall provide the justification report.
-
- Till the further solution is achieved, existing methods shall be used for analysis.
-
- Based on ADD’s justification report, Quality Control -Head or Designee shall make an entry in the register maintained at the location and intimate the closed status to CQ-designee against the respective pharmacopoeial change number.
-
- If as per the evaluation report (where methods are evaluated) the product passes, the plant shall revise the STP as per the pharmacopoeial monograph.
-
- The plant shall revise the STP/Specification as per new pharmacopeia and shall make it effective as per pharmacopoeial change requirement
7.0 Annexures – SOP for Handling of Pharmacopoeial Change
Annexure-1: Guideline for Applicability of Pharmacopoeial Changes as per Market Requirement.
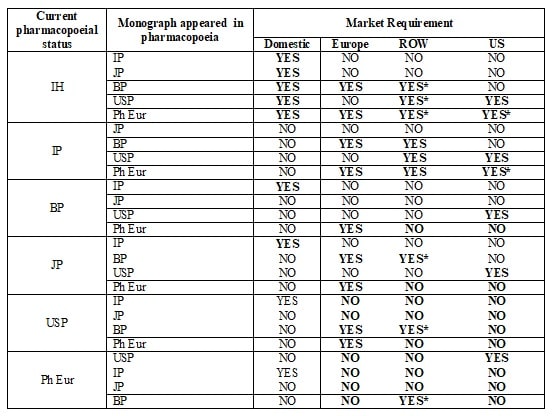
*Note: Product to be evaluated for compliance to the monograph. RA shall decide the requirement of changes.
Annexure-2: Format of Register for Handling Pharmacopoeial changes Maintained at the Location
|
CQ Allocation number |
Location Allocation number | Impacted Item
(FP/API) |
Name of Product | Scope | Current Pharmacopoeial status | Version no of New
Pharmacopeia |
The effective date of a pharmacopoeial monograph |
|
Analysis completed on |
STP code/date | Artwork updated on | FDA license received on | Change control closure date | Implemented (Yes/No/NA) | Remarks |
Status |
Annexure-3: Format for the Pharmacopoeial Change Evaluation Report
| Product Code | |||
| Name of Product/ Brand name | |||
| Generic name | |||
| Current Pharmaceopoeial status | |||
| Evaluation against pharmacopeia with version no and effective date | |||
| Batch number of evaluation samples | |||
| BMR No | |||
| Pharmacopoeial change No | |||
| Current document No and effective date | |||
| Changes appeared in the current pharmacopeia | |||
| Action to be taken | |||
| Evaluated By/date Verified By (Head-QC)/Date: | |||
| Attachments | : | Compilation of results
Initial COA ( if the results derived directly due to the same methodology). Others |
|
| Final Conclusion | : | ||
| Justification if any | : | ||
| If implemented, revised document no and effective date | : | ||
| Prepared By/date: Checked By/date: Approved By/date: | |||
| Known impurities comparison table | ||
| Sr # | Nomenclature as per Current status | Nomenclature as per Phamacopoeial monograph |
Compilation of results
| Sr No | Name of test | Limits as per current specification | Limits as per Pharmacopoeia | Observation | Remarks | Test to be adopted for revision | |
| Initial result | Results as per pharmacopeia | ||||||
Conclusion:
Annexure-4: Checklist for Evaluation of Impact Analysis of Pharmacopoeial Change.
|
Sr. No. |
Particulars |
Impact analysis |
| 1. | Inclusion of monograph in Pharmacopoeia |
|
| 2. | Revision in General monograph
i.e. General procedure change |
|
| 3 | Revision in General Notices |
|
Sr# |
Particulars |
Impact analysis |
| 4. | Omission of monographs |
|
| 5. | Change in Specification |
|
| 6. | Change in Methodology |
|
| 7. | Omission of test |
|
| 8. | Addition of test |
|
| 9. | Change in storage |
|
| 10. | Change in Labeling |
|
Sr. No. |
Particulars |
Impact analysis |
| 11 | Change in characteristic e.g. Critical physical properties |
|
| 12. | Change in Title |
|
| 13. | Change in content statement e.g. Injection to “Ready to use injection” |
|
| 14. | Inclusion or Revision of “Warning” |
|
Annexure-5: Pharmacopoeial Change Handling Flowchart.
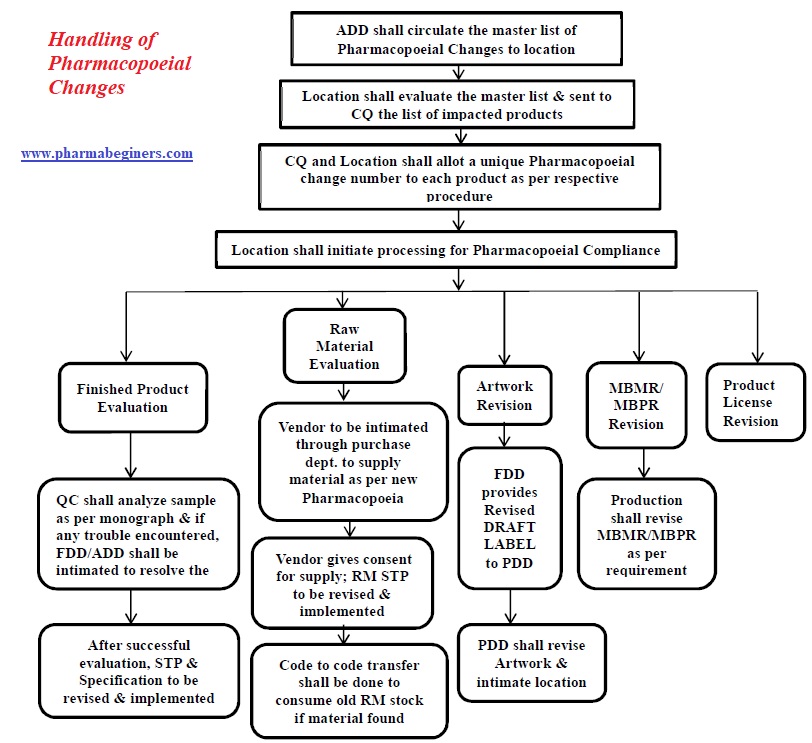
Annexure-6: Individual Product Impact Assessment cum Closing Report.
| Individual Product Impact Assessment cum closing Report | |||||||
| Name of impacted Product/Material: | |||||||
| Reason: | |||||||
| CQ Allocation no. : | Location Allocation no. : | ||||||
| Impacted Dept. | Impacted Items | QA Assessment | HOD (Sign/Date) |
Tentative Compliance Date | Effective Doc. No. | Closure Compliance | |
| QC | 1). Chemical Evaluation | ||||||
| 2). Revision of STP/Spec. | |||||||
| PDD | 1). Revision of Artwork | ||||||
| PMS | 1). Receiving status of new PM | ||||||
| 2) Destruction status of Old PM | |||||||
| RMS | 1) Receiving status of new RM | |||||
| 2) Destruction status of old RM (If required) | ||||||
| 3).Code to Code transfer status of impacted RM (If applicable) | ||||||
| Production | 1).Revision of impacted MBMR | |||||
| 2). Revision of Formulation (If required) | ||||||
| Packing | 1). Revision of impacted MBPR | |||||
| QA | 1). Updation of Product License | |||||
| 2). Revised Draft label status |
Annexure-7: Impacted Master List.
| Assessment for Pharmacopoeia | |
| Effective Date for Pharmacopoeia |
| Sr. No. | Location change Allocation No. | Impacted Item (API/FP) |
Name of Item | Impacted Brands | Current Generic Name | Current Label Claim | New change in Pharmacopoeia |



Pingback: Analytical Method Transfer (USP 1224) Guideline - Pharma Beginners