Standard Operating Procedure for Qualification, Handling and Usage Of Reference Standards, Calibration Standards, Impurity Standards, Working Standards, and Working Standard Solvents.
SOP for Laboratory Standard Management
1.0 PURPOSE:
-
- The purpose of this SOP is to describe the procedure for qualification, handling, storage and usage of reference standards (RS), calibration standards, impurity standards (IMS), working standards (WS), working standard solvent (WSS) in the quality control department.
2.0 SCOPE:
-
- This SOP is applicable for qualification, handling, storage and usage of reference standards, calibration standards, impurity standards, working standards and working standard solvent in the Quality Control department at pharmaceuticals manufacturing plant.
3.0 REFERENCES:
-
- Guideline for the establishment, handling, storage, and use of chemical Reference substances (National Pharmaceutical Control Bureau).
-
- SOP for Analytical Master Document Programme.
-
- SOP for Sampling Procedure of Raw material.
4.0 RESPONSIBILITY:
-
-
Analyst :

-
-
- Carry out the analysis as per the procedure.
-
- Verify the standards COA and container which is procured from outside.
-
- Maintain the inventory logbooks.
-
- Distribute the material prepared in the respective bottles with labeling.
-
- Keep and replace the correct standard bottle as per the validity in the storage chamber.
-
- Fill the checklist and verify the container for the impurity standard and reference standard.
-
- Qualification, handling, storage and usage of standards as per the procedure.
-
- Keep all the standards used in the QC lab securely under lock and key.
-
- Record and maintain all the raw data as per the procedure.
-
-
Section Head or designee :
-
-
- Ensure the availability of the standard.
-
- Initiate for standard or material procurement.
-
- Ensure that all the standards used in the QC lab are kept securely under lock and key by the designed person.
-
- To ensure the handling, storage, and usage of the standards as per the procedure.
-
- Ensure that the documentation is done as per the procedure.
-
-
Head Quality Control or designee :
-
-
- Check the SOP.
-
- Ensure that the system and procedures are followed as per the SOP.
-
- Ensure that the documentation is done as per the SOP.
-
- Co-ordination for the procurement of the new standards.
-
- Review of schedule prepared for qualification.
-
- Ensure that the training is given to the concerned persons be Implementation of the SOP.
-
-
Quality Assurance:
-
-
- To check the SOP.
-
- To ensure the implementation of the system as per SOP.
-
-
Quality Head and Plant Head :
-
-
- Quality Head and Plant Head shall be responsible for review and approve the SOP.
4.0 ABBREVIATIONS USED IN SOP FOR WORKING STANDARD QUALIFICATION:
-
- ADD: Analytical development department
- API: Active pharmaceutical ingredient
- CDL: Central Drug Laboratory
- COA: Certificate of Analysis
- DSC: Differential Scanning Calorimeter
- GRS: Global Reference standard
- IMS: Impurity Standard
- LOD: Loss on Drying
- MSDS: Material Safety Datasheet
- NMR: Nuclear Magnetic Resonance
- PD Lab: Process Development Laboratory
- R&D: Research and development
- RS: Reference standard
- WS: Working standard
- WSS: Working standard solvent
- XRD: X-ray Diffraction
5.0 DEFINITION OF TERMS – SOP FOR WORKING STANDARD QUALIFICATION:
-
-
Reference standard:
-
-
- Reference Standard is pharmacopoeial standard material (BPCRS / USPRS or from any other pharmacopeia) that is intended for use in specified chemical and physical tests, in which its properties are compared with properties of samples under examination and is used for qualification of the working standard.
-
-
Working standard :
-
-
- Working Standard is a material that is intended for use in routine analysis of API, Intermediate and raw material, in which its properties are compared with properties of samples under examination.
-
- The working standard is qualified against in-house reference standard or pharmacopoeial reference standard.
-
-
Impurity Standard:
-
-
- Impurity Standard is a material that is intended for use in identification / Estimation of impurity in drug substance/drug product.
-
-
In- house reference standard (Non-pharmacopoeial) :
-
-
- A Chemical substance/material that is being issued by the R&D which intended for use in specified chemical and physical tests, in which its properties are compared with properties of samples under examination and is used for qualification of the working standard.
-
-
Working standard solvent:
-
-
- Working Standard of solvent is a material that is intended for use in routine analysis of Solvents, in which its properties are compared with properties of samples under examination.
-
-
Calibration Standard :
-
-
- The standard which is used for calibration of Laboratory Instrument /Equipment.
6.0 PROCEDURE FOR WORKING STANDARD MANAGEMENT:
-
- Source: Working standards, Reference Standards, Impurity standards, and Working standards solvent shall be sourced from the different authentic vendors with proper certification.
-
- Sources of Reference standards, Calibration standards, and Impurity reference standards :
- Reference standard and calibration standards can be procured from pharmacopoeial agencies. like…
-
-
- British Pharmacopoeia Commission Laboratory.
-
-
-
- WHO Collaborating Centre for Chemical Reference Substances.
-
-
-
- The United States pharmacopoeial Convention, Inc.
-
-
-
- European Department for the Quality of Medicines.
-
-
-
- Indian Foundation For Pharmaceuticals Reference Standard
-
-
-
- Central Drug Laboratory (CDL).
-
-
- Reference standards (which are non-pharmacopoeial, well-characterized and qualified) shall be procured from R&D along with the COA or from the originated vendor.
-
- Calibration standards can be procured from authentic sources like Fluka, Merck, Sigma Aldrich, Lancaster or any other authentic source with certification.
-
- It shall be either traceable to NIST or pharmacopoeial reference standards.)
-
- Impurity standards shall be procured from R&D, material vendor or from pharmacopoeial agencies, Fluka, Merck, Sigma Aldrich, Lancaster or any other authentic source with certification.
-
- Before reprieving any reference standard, working standard, impurity standard, etc. temperature, storage, dispatching conditions shall be checked physically.
-
- The material shall be received only if the condition is found satisfactory, otherwise, it shall be returned back to its source.
-
-
Source of Working Standard and Working Standard Solvent:
-
-
- For working standard (WS) preparation, consignment of good quality (approved material) shall be selected by analyst based on LOD, impurity (should be as less as possible) and Assay (Shall contain highest potency i.e near to 100% or 1000 mcg) or closest to highest purity from available stock at plant.
-
- If approved material is not in stock, available existing consignment can be qualified as WS along with the routine analysis.
-
- In case of unavailability of base material for WS preparation, it can be procured from the material vendor or manufacturer’s WS (along with the COA) that can be used for the analysis.
-
- For the qualification of Working standard solvent, HPLC/GC (if available) or any high purity chemicals from Aldrich, Fluka, Lancaster, Merck or any other authentic source shall be procured (with certification).
-
-
R&D :
-
-
- Organic synthesis shall synthesize the material and qualifies with the help of the Analytical Development Department as reference standard as per requirement.
-
- As per the requirement of the plant, organic synthesis provides the respective qualified reference standard and GRS to the concerned location along with approved COA.
-
- The GRS shall be supplied to all user locations for common usage.
-
-
Reference Standard, Impurity Standard & Calibration Standard lots and evaluation:
-
-
- Reference standards, calibration standards received from pharmacopeia or R&D, shall be verified against the COA (if applicable), supplied by respective agencies.
-
- Updated details (lot changed) of Reference Standards shall be verified (At respective frequency) on respective Internet site (or against catalog) and accordingly usage and procurement of reference standard shall be planned by user.
-
- Verify the Reference standards, calibration standards and impurity standards received from other locations or by the vendor against the provided certificate.
-
-
Working Standard / Working Standard Solvent lots and evaluation :
-
-
- The WSS evaluation to be done at the location with respective solvent specifications against a current lot of reference standards.
-
- The WS evaluation to be done at the location with respective API specifications against the current lot of reference standards provided by the R&D.
-
-
Analysis and Qualification of Working Standard:
-
-
- The analyst shall sample the material which is selected for the WS preparation as per “General sampling procedure for Raw material”
-
- The analyst shall take the required quantity of material, collect it in two different polyethylene zip lock bags (or in the relevant container), one for the analysis and second for the final WS usage.
-
- In the case of hygroscopic or light-sensitive material sampling follow specific instruction as per material characteristic.
-
- The analyst shall carry out the test as per the current test procedure, against the reference standard as per Annexure -1.
-
- After completion of the analysis, the analyst shall get the WS Code no. allocated from designated QC person and using this code no., prepare the COA of WS as per Attachment-10 and attach the raw data with COA or give the reference of raw data.
-
-
Note: If LIMS COA is not available then prepare manual COA for WS/WSS as per Annexure-10.
-
-
- The analyst shall submit the COA along with raw data for the checking to the designated person.
-
- After completion of checking, the checker shall make initial/date in the “Checked By/Date” column and if found satisfactory forward it to Head QC or Designee for the approval, who shall make initial/date in “Approved By/ Date” column.
-
- The analyst shall handover the material from the second container to the designed person.
-
- In case the specification is revised with the change of limits due to the change in method of analysis, update the working standard protocol.
-
- In case the change in the limit, confirm the results of working standard COA against the revised specification. If the results are within the limit, prepare an amendment COA.
-
- If the method of analysis has been changed, analyzed the WS for that specific test with an update method and prepare an amendment COA.
-
-
Analysis and Qualification of Working Standard Solvents:
-
-
- The designed person shall arrange for the procurement of high purity analytical grade solvent with minimum pack size along with COA.
-
- For the working standard solvent, if the Purity is greater than or equal to 99.0% (as per certificate of the manufacturer), shall be directly used as WSS.
-
- If the Purity of the solvent is less than 99.0% given by the manufacturer then qualification is required.
-
- For the qualification of WSS appropriate in-house method (Method can be applied which is used for routine sample analysis or any other validated method) or,
-
- The method of analysis mentioned for chemicals and reagents in the Pharmacopoeia like USP, BP, IP, etc. shall be taken and it’s analytical test procedure shall be finalized accordingly.
-
- The analyst shall carry out the test, as per Annexure -1 but not limited too.
-
- After completion of the analysis, COA shall prepare as per Annexure-10, attach the manufacturer’s COA and Raw data with COA.
-
- The analyst shall submit the COA along with raw data for the checking to the designated person,
-
- After completion of checking, the checker shall make initial/date in the “Checked By/Date” column and if found satisfactory forward it to Head QC or Designee for the approval, who shall make initial/date in “Approved By/ Date” column.
-
-
Coding and Labelling and distribution of standard:
-
-
- The concerned person shall distribute the material from the second container (which is sampled for WS usage) – in two parts.
-
- Distribute first part equally, into twelve previously cleaned and dried containers (Amber color USP type I borosilicate glass bottles with Bakelite screw cap) and the second part in one separate bottle identified as “Mother container”. Label them with the verified label.
-
- Note-1: Depending on usage and sensitivity of the material, more bottles can be prepared (Also single time usage Working Standard bottle can be prepared).
-
- If the Working Standard of all the twelve containers exhausted, fill the required quantity in another bottle from the “Mother container” and put it for use.
-
- Note-2 :(Cleaning procedure for WS or WSS container) Soak new containers for 4 to 5 hours in 1%v/v HCl or 1% v/v HNO3 before washing.
-
- Transfer the containers into the Teepol/detergent solution.
-
-
Or follow the SOP for Laboratory Glassware Cleaning.
-
-
- Again rinse the containers thoroughly with running potable water.
-
- Finally, rinse the container thoroughly with purified water and transfer it into a drying oven at a temperature between 50ºC to 60ºC.
-
- Before starting the analysis, allot the unique code no. of Working Standard /WSS/Calibration /Impurity/Reference standard as follows.
-
-
For Working Standard/Working Standard Solvent coding system :
-
-
- Working standard Code No. : WS-YY-XXX or WSS-YY-XXX
Where,
WS = Identification for the “Working Standard”
WSS =Identification for the “Working Standard solvent”
YY = Last two digit of current Year
XXX = Serial No. of prepared standard for the current year.
-
- Example: If the first Working standard prepared in the year 2020 then WS code no. Shall be as follows WS Code No.: WS-20-001
-
-
The coding of the Reference standard :
-
Reference standard Code No.: RS-YY-XXX
Where,
RS = Identification for the “Reference standard”.
YY = The last two digits of the current Year.
XXX = Serial No. of a received Reference standard for the current year.
-
- Example: If the XYZ reference standard is first received in the year of 2020, then Reference standard Code No.: RS-20-001
-
- The coding system shall be continuing in serially.
-
- If the same lot of Reference Standard received again, give the same code No., make an entry for the quantity, received By/Date in the Annexure -5.
-
- Put initial in the prepared by column for the same checklist (Previously filled) for the Reference/calibration/Impurity Standard (Annexure -2),
-
- After a satisfactory review. The coordinator shall keep the received document with the same checklist form.
-
-
The coding of the Impurity standard.
- Impurity Standard Code No.: IMP-YY-XXX
-
Where,
IMP = Identification for the “Impurity standard”.
YY = The last two digits of the current Year.
XXX = Serial No. of received Impurity standard for the current year.
-
- Example: If the Lactum Impurity standard is first received in the year 2020, then Impurity standard code no. IMP-20-001
-
- The coding system shall be continued serially.
-
- If the same lot of Impurity standard received again, give the same code No., make entry into Annexure -5) and put initial in the same checklist (Previously filled) for the Reference/Impurity Standard (Annexure -2)
-
- After a satisfactory review. The coordinator shall keep the received document with the same checklist form.
-
-
Coding of calibration standards Coding of Calibration standard :
-
-
- Calibration standard Code No.: CAL / XX / YYY
Where,
CAL = Suffix for the “Calibration standard”.
XX = Name of Calibration Standard
YYY = Serial No. of Calibration standard
-
- Example: If Holmium oxide is first received Calibration standard, then Calibration standard Code No.: CAL / Holmium oxide / 001
-
- Designed person shall put label on WSS and WS as per Annexure–8 and Annexure –9.
-
- The bottle with the verified label (Shall review by the second person and put initial and date) and keep it for usage at the designated place and make an entry in Working Standard and Working Standard Solvent logbook (Annexure -3 and -4 respectively) and update the list as per respected Annexures (Annexure -15).
-
- For WS, WSS, Reference Standards, the designed person shall verify the content of label (as per respective Annexures -7, -8, and -9), if required details are missing, re-label with required contents. The Reference standards / Impurity standards shall be kept in a designated places and update the list of standards (Annexure-15, 17).
-
- As per allotted unique code no. of standards, fill the details in the label as per respective standards label, and paste it on bottle/container.
-
- The Designed person shall prepare required labels (as per Annexure -7,-8, -9,-13 for respective standards), the designated person shall verify the label and put initial and date in the label against verified.
-
- In label following content to be filled for
-
-
Working Standard Bottle no.:
-
-
- Bottle Identification number as per distribution like 1/12, 2/12, 3/12………. 12/12.
-
- Storage condition:
-
- Maintain the storage conditions as per the pharmacopeia / Reference standard or base on any other authentic source data (Like: Merck Index).
-
- Direction for use: Instructions for usage like, Determine the water content before use/dry before use should be mentioned on the label (If required).
-
- Use before ( Bottle):
- The validity of one vial is for one month. e.g. If WS is effective from 21.03.2015 and it’s first container opening date is 21.03.2015 then mentioned the use before date as 21.04.2015.
-
- Designed QC person shall paste the labels for calibration standards as per Annexure-13.
-
-
Handling and Usage of Working Standard:
-
-
- For Qualified WS/WSS, the Designed person shall keep only one bottle for the usage in the storage chamber and rest as reserved containers separately.
-
- Made entry in Working Standard and Working standard solvent logbook (Annexure–3 & 4 respectively).
-
- Prepare a list as per Annexure – 15 with respect to opened WS/WSS bottle No. and display the same beside storage chamber.
-
- The concerned person shall enter details in WS template issuance register as per Annexure-20 and issue the template to respective analyst.
-
- To use the qualified solvent during routine use for the analysis transfer the quantity slightly more than the required quantity in a clean beaker/suitable container to avoid any contamination of mother container. Discard the leftover quantity.
-
- If the working standard solvent is contaminated before the date of “use before date”, or consumed before the valid date. Qualify the new solvent standard and destroy the contaminated standard, if it impacts the purity of an interesting peak.
-
- At the time of any Standards received, the designed person shall fill the checklist by referring MSDS, COA and other relevant documents.
-
- Make an entry for Reference standards, Impurity standards and Calibration standards in the “Reference/Calibration/Impurity standard inward register ” (Annexure -5) and for calibration standards.
-
- Update the list of calibration standards as per Annexure -11.
-
-
Note: Prepare an index of Reference / Impurity/ Calibration Standard along with page no. for traceability.
-
-
- Designed person shall put initial and date on satisfactory review, in the Prepared by and Date column of the checklist, handover the data to the designated person for reviewing.
-
- Designed person write In-House code no. on the standard which is received from outside and COA shall be signed with the date.
-
- On receipt of Reference/Impurity standards, the reviewer shall review the data and make entries in the “Reference/Calibration/Impurity standard inward register” (Annexure -5).
-
- On satisfactory review, put initial and date in the reviewed by and date column, shall handover the data to the concerned person.
-
- Record the details of consumption records in the Reference/Impurity Standard usage logbook (Annexure -6).
-
-
Maintain the consumption of calibration standards as per Annexure-12.
-
-
- The Reference Standard / GRS shall be provided by R & D dispensed in multiple containers for single-time use, which should be opened in humidity controlled area.
-
- In case of any abnormal observation found in the physical condition of the container, the appearance of Reference standards/Impurity standards, the analyst shall inform to Head QC or designee and then discard it and procure the new Reference Standard/Impurity Standard.
-
- If any container got exhausted or broken during handling then the next container shall be issued with remark and a new container shall be made at the end, from the “Mother container”.
-
-
At the time of every usage of standards close the container tightly, and wrap the para-film or by other means, to protect it from moisture.
-
-
- If the Impurity standard is not available or insufficient quantity for the analysis, the stock solution of the Impurity standard can be used for analysis purposes with appropriate “Solution Stability Data ”.
-
- For pharmacopoeial standard, if valid use before the date provided in the revised catalog then mentioned the use before the date of that standard in the inward register and update the label of the bottle.
-
- If WS is prepared for the qualitative purpose a note on the label shall be mentioned as “For qualitative use”.
-
- Standards crossed the use before the date and are qualification COA is required from outside shall be removed from usages and shall be kept in lock & key and status shall be documented in remarks column as “Remove from usage”. After receipt of the revised COA, the subjected standard shall be reinverted.
-
- In case assay values are reported by more than one method then the strategy of purity consideration shall be in the following order.
-
-
- Elemental analysis
-
-
-
- Titrimetry
-
-
-
- HPLC/UV
-
-
-
Consideration :
- If the material is not having good quality can be used after purification at PD Lab/R&D as per the respective process.
-
-
- Purification records shall be maintained by the PD lab/R&D.
-
- The same shall be analyzed completely.
-
- If drug Product or drug substance has been transferred from other locations, the analyst shall use the WS, IMS or RS provided by the first location after the satisfactory review of COA against the applicable specification.
-
-
In case WS potency is more than 100%, potency shall be considered as 100% for the calculation and the same value shall be reflected on the WS bottle label.
-
-
- A new working standard shall be prepared and qualified against the current lot of In house reference standard or pharmacopoeial reference standard before the expiry date of the current WS.
-
- Note: For convenience, Working Standard qualification activity shall be initiated 30 days prior to use before the date of current WS, so that qualified WS is available.
-
- If the Working Standard is required for the qualitative purposes, approved material shall be used confirming that it is appropriate for the intended use.
-
- The analyst shall calculate the results after performing the test and check against the limit.
-
- If results is found out of specification, inform to Head QC or designee and follow the “out of specification” SOP for the investigation of the failure.
-
- Note: During the qualification of WS/WSS, if the verification criteria (eg. : RSD, four individual results are not matching with approved material taken for the qualification, etc.) does not meet such case shall be handled through “repeat analysis SOP.
-
- If a sufficient quantity of reference standards, Impurity standards, and Working standards are not available then weight of reference standards, impurity standards, and Working standards can be adjusted in such a manner, that the final concentration of reference solution, Impurity solution and Working standard solution should remain unchanged.
-
-
Re-qualification of Working Standard:
-
-
- If the fresh consignment of good quality is not available and sufficient quantity of WS remaining, the analyst shall re-evaluate the same Working Standard after the authorization of Head QC or designee at the plant.
-
-
Storage and validity of Reference/Working Standards :
-
-
- All the standards should be kept securely under lock and key by the designed person and shall be stored as per the storage requirement of the individual standards.
-
- Store the standards as per their respective storage condition, specified on the standard vials or catalog. Unless otherwise standards should be stored at a temperature between 2°C to 8°C.
-
- If the substance is hygroscopic store the same substance in desiccator or cabinet having suitable desiccant (e.g. Silica gel).
-
- The designed person shall activate the silica gel as and when required.
-
- If Working Standard is unstable or light-sensitive store the same as per their specified storage condition.
-
- If the storage conditions of light-sensitive and unstable standards are not mentioned then store at 2°C to 8°C.
-
- During the storage of light-sensitive standard, the standard should be kept in amber color vial and vial should be wrapped with black poly bag.
-
- The analyst shall also check the temperature and humidity of the standards storage area daily to ensure that standards are properly kept to avoid deterioration.
-
- If Storage condition mentioned on Reference Standard (In-house / pharmacopoeial Reference standard) and Working standards then storage condition shall be mentioned on Reference standards and WS container label.
-
- The working standard shall be valid for the period of one year from the effective date of Working standard or up to the expiry date/retest date of material whichever is earlier.
-
- Use before date shall be assigned as one month from the date of bottle opening to each prepared bottle, which should not be more than “use before date” mentioned on COA.
-
- The validity can be extended up to 2 years where supportive stability data are available.
-
-
Note Material’s stability data up to 24 months should be procured from the vendor.
-
-
- Storage conditions for calibration standards shall be mentioned as per pharmacopeia or base on any other authentic source data (Like Merck Index).
-
- If the storage condition is not available from any other authentic source data (Like Merck Index), the calibration standard shall be stored below 25°C and the label for the same shall be affixed on the container.
-
- Pharmacopoeial Reference Standard / Impurity Standard shall be used until the validity of the current lot.
-
- The validity for the calibration standard is for two years from the date of opening or as recommended by the manufacturer, whichever is earlier.
-
- Working standard Solvent (WSS) shall be valid for the period of two years from the effective date of WSS or up to the expiry date/retest date of material whichever is earlier.
-
- If the expiry date is not available for WSS which are not qualified (purity is greater than or equal to 99.0%), WSS shall be valid for two years from the date of opening.
-
- Impurity/Reference Standard/GRS provided by the R & D, the validity shall be as per certificate.
-
Tracking system for In-house standard:
- Prepare a list in an excel spreadsheet for standards with expiry or due for re-qualification details.
-
- The standards details which will fall under expiry before two months shall be put out.
-
- The list shall be reviewed and fill the request details in the Impurity/ Reference standard request register as per Annexure-14, to get the standard prior to expiration.
-
- The designed person shall procure the pharmacopoeial standard through the purchasing department.
-
- Those reference standard which gets exhausted at the time of analysis, the analyst shall communicate to the concerned person for procurement of standards.
-
- On receipt of the standard, the status shall be updated in the Impurity/Reference standard request register (Annexure -14).
-
- Expiry Date of Standard:
| Name of Standard | Expiry Date | Comments |
| Working Standard | One Year | Valid for the period of one year from the effective date of Working standard |
| One Month | The designed person shall give the use before date one month to each prepared bottle from the date of the bottle opening. | |
| Pharmacopeias Reference Standard | Till the validity of the current lot | None |
| Name of Standard | Expiry Date | Comments |
| Working Standard Solvent | Two Year | Valid for the period of two years from the effective date of Working standard solvent. |
| Impurity standard provided by the R&D | As given by R&D | As per certificate. |
| Pharmacopeias Reference Standard | Till the validity of the current lot | None |
-
-
Destruction of Standards (Working Standard/Calibration Standard):
-
-
- Destroy the Expired / Contaminated standards as per SOP of Destruction of Excess sample after analysis.
-
- The analyst shall make a cross line on the bottle label.
-
- Transfer the material into a bucket containing water.
-
- Analyst shall stir the solution with the help of a glass rod and allow dispersing the material.
-
- After the complete dispersion, drain the dispersion into the drainage.
-
- The designed person shall discard the bottle of WS at each month of interval (on the date of use before) and replace it with the next WS bottle, which is valid for another one month period.
-
- Discard the mother container along with the last bottle of respective working standard and make the entries in Annexure -3
-
- In case of any deterioration is found in any bottle replace it with the next No. of the bottle and record it.
-
- Corrective and preventive action to be taken regarding storage and handling and verification of results of products analyzed using that deteriorated standard to be done on a worst-case basis.
-
-
General precautions for usage of standards:
-
-
- Replace the indicating type silica if any whiteness observed. (original color is dark blue).
-
- The analyst shall check the temperature/humidity of the chambers once in the day and fill in Annexure-19. If observed any abnormality immediately informs to head QC or designee.
-
- At the time of use, the analyst is to ensure that the standard is not expired.
-
- During weighing, the analyst should keep the standard out of the recommended storage condition for as short a time as possible to prevent light and moisture from affecting the standard.
-
- To avoid condensation of moisture, refrigerated standards must be allowed to come to room temperature, wiped off with tissue paper, before the container is opened for weighing.
-
-
After the analyst has weighed the standard, it is to be returned to the appropriate storage location.
-
-
- After taking the desired quantity of WS/WSS, the analyst shall make an entry in the WS/WSS Usage Log Book as per Annexure-21.
-
- Dry the standards in accordance with the labeling (Directions for use). Do not dry the standard in the original container.
-
- Transfer a portion of the material to a separate drying vessel.
-
- Do not return the dried standards to the original bottle in order to avoid any possible contamination of the stock.
-
- After drying, place the dried standard into the desiccator to equilibrium before weighing.
-
- In accordance with the labeling (directions for use) if the standard should be used with a correction for the water content or the loss on drying, determine on a separate portion of material at the time a standard is to be used ( If required ).
-
- The qualified material if changes the color or description then this standard shall not be taken for further analysis.
-
- Do not return the remaining quantity of standards (RS/Impurity/WS) in the respective container, after weighing.
-
- For hygroscopic materials, ‘one time use’ working standard vials shall be prepared.
7.0 ANNEXURES:
Working standard /Working standard solvents qualification parameters. (Annexure 1)
A) Test – Description
-
- Parameter – Physical
-
- No. of Sample Preparation – Single
-
- No. of Analyst – Single
-
- Evaluation Parameter – Should meet the requirement of the raw material Specification.
B) Test – Identification
-
- Parameter – Physical
-
- No. of Sample Preparation – Single
-
- No. of Analyst – Single
-
- Evaluation Parameter –
- IR – Should correspond to the reference spectrum or spectrum obtained from the RS or should meet the material specification.
- Evaluation Parameter –
-
-
- UV Spectrophotometer – Should correspond to the reference UV graph or as per the respective specification.
-
-
-
- TLC – Should correspond to the standard solution or should meet the material specification.
-
C) Test – Loss on Drying / Water Content
-
- Parameter – Loss of solvent or water evaporation On drying or Water content present in the sample.
-
- No. of Sample Preparation – Duplicate
-
- No. of Analyst – Single
-
- Evaluation Parameter – Should meet the requirement of the respective Specification Not more than 0.5 % variation. Report the mean value in the certificate of working standard.
D) Test – Chromatographic Purity / Related Substances
-
- Parameter – To check the materials Chromatographically pure or presence of Substance-related to the Material under analysis by HPLC or TLC.
-
- No. of Sample Preparation – Single
-
- No. of Analyst – Single
-
- Evaluation Parameter – Should meet the requirement of the respective Specification.
E) Test – Assay (BY HPLC / Titrimetric)
Note – In the case where assay by titrimetry and HPLC both exist– HPLC to be followed for Qualification.
-
- Parameter – To check the Purity of the Material under analysis.
-
- No. of Sample Preparation – Four (one standard and two sample preparation in each set )It should be two independent analyses.
-
- No. of Analyst – Two (Each set of analysis performed by a single analyst)
-
- Evaluation Parameter – Mean and individual Potency of ‘four analysis should meet the requirements of the specification. RSD % of ‘four results’ should not more than 1.0%. Report the mean value in the Certificate of analysis of the working standard.
F) Test – Assay or Chromatographic purity by GC for WSS)
-
- Parameter – To check the Purity of the Material under analysis.
-
- No. of Sample Preparation – Four (one standard and two sample preparation in each set )It should be two independent analyses.
-
- No. of Analyst – Two (Each set of analysis performed by a single analyst)
-
- Evaluation Parameter – Mean and individual Potency of ‘four analysis should meet the requirements of the specification. RSD % of ‘four results’ should not more than 1.0%. Report the mean value in the Certificate of analysis of the working standard.
Checklist for Reference Standards / Calibration standard / Impurity Standards. (Annexure 2)
(Fill at the time of receipt)
Name of Reference/calibration/Impurity Standard:_________________
Reference Pharmacopoeia/Make : (USPRS / BPCRS / EPCRS / In-House/……………)
Lot No./ B No.:_________
In house code no of the standard : _______________
| 1.MSDS available. |
Available/Not available |
| 2. In case of In-house standard received, verify the detail on container against COA. It should match. |
Satisfactory/Not satisfactory |
| 3.Lot No./ B No. as per the current requirement. (In case of pharmacopoeial standard.) |
Satisfactory/Not satisfactory |
| 4. Verified the potency and Use before date. |
Satisfactory/Not satisfactory |
| 5. Copy of Purchase proof/Delivered note attached. |
Received/Not received |
| 6.Condition of the container. |
Satisfactory/Not satisfactory |
Space to affix the label:
Received by & Date:_____________ Reviewed by & Date:__________
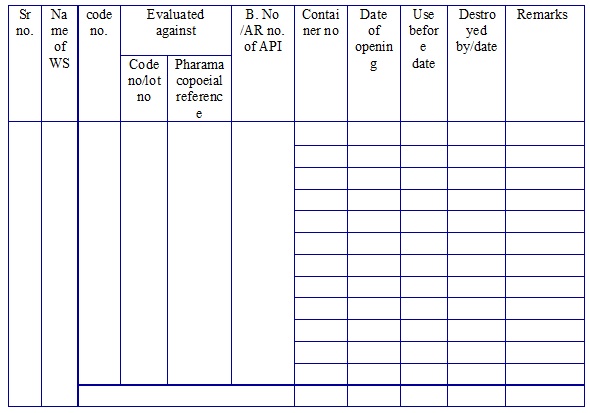
Working Standard log book. (Annexure 3)
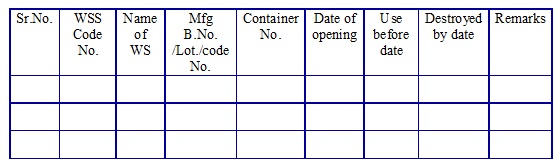
Working standard solvent log book. (Annexure 4)
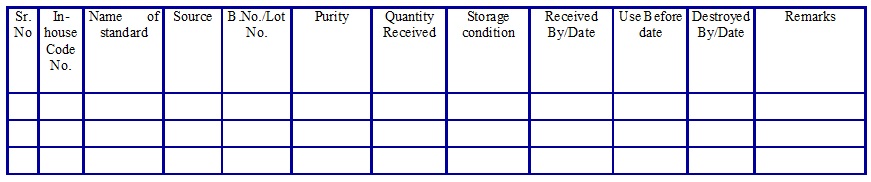
Reference/Calibration/Impurity Standard Inward Register. (Annexure 5)
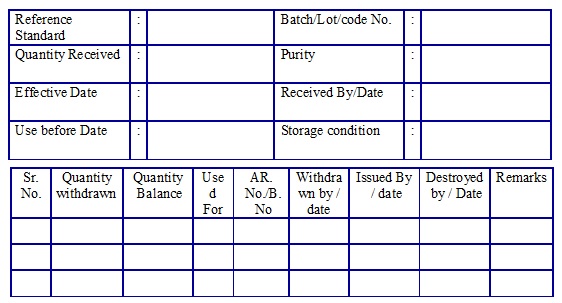
Impurity/Reference Standard Usage log book. (Annexure 6)
Reference / Impurity Standards label. (Annexure 7) – Prepare as per SOP for the Labeling system.
Working Standard of the solvent label. (Annexure 8) – Prepare as per SOP for the Labeling system.
Working standard label. (Annexure 9) – Prepare as per SOP for the Labeling system.
COA of Working Standard / Working Standard Solvent. (Annexure 10)
| CERTIFICATE OF ANALYSIS
WORKING STANDARD/WORKING STANDARD SOLVENT |
||||
| Material | ||||
| WS No. | AR No. | |||
| Spec Code | Revision No. | |||
| Evaluated against | LOT No. / Batch No. / Code No. Valid up to date | |||
| Mfg. Date | Exp. date | |||
| Effective Date | Use before date | |||
| Quantity prepared | No. of containers Prepared | |||
| Direction for Use | ||||
|
TEST |
RESULT |
SPECIFICATION |
||
| Description | ||||
| Identification | ||||
| LOD/Water | ||||
| Related compounds /
Chromatographic purity |
||||
| Assay (as is basis) (on dried/Anhy. basis | ||||
| Remarks: The substance complies/does not comply against the standard and qualifies /
does not qualify for usage as a working standard. |
|||
| Storage: As per the Requirement. | |||
| H. B. No./Template No. | Page No. : | ||
|
Analyzed by / Date |
Checked by / Date |
Approved by / Date |
|
List of the calibration standards. (Annexure 11)
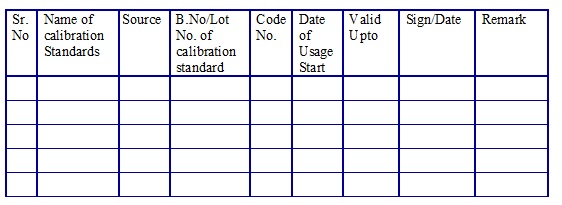
Calibration Standards Usage Log. (Annexure 12)
| Name of Calibration Standard | ||||||
| Batch/Lot No. | Make : | |||||
| Received Quantity | Manufacturer Expiry Date : | |||||
| Purity | Valid Upto : | |||||
| Code No. | ||||||
| Sr. No. | Date | Quantity withdrawn | Used for | Sign/Date | Remarks | |
Calibration Standard label. (Annexure 13)
| CALIBRATION STANDARD | ||
| Name | : | |
| Code No. | : | |
| Opened by/Date | : | |
| Use before | : | |
| Storage Condition | : | |
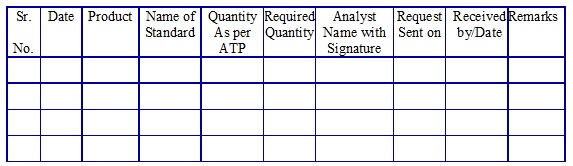
Impurity/Reference/Calibration standard request register. (Annexure 14)
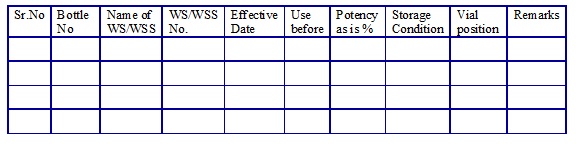
WSS/Working Standard list. (Annexure 15)
Impurity Standard list. (Annexure 16)
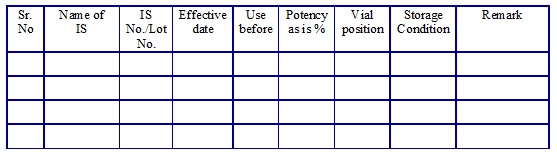
Reference Standards list. (Annexure 17)
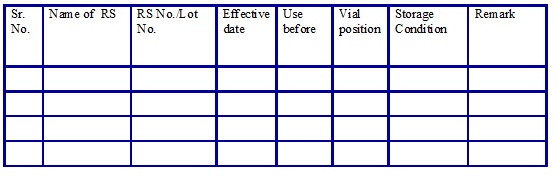
Checklist for working standard COA. (Annexure 18)
Working standard name: Code No. :
| 1. Reference standard or WS and respective No. | |
| 2. Mfg. date and exp. date of the material used for the WS preparation | |
| 3. Approved material COA | |
| 4. Expiry period of the RS or WS. | |
| 5. Ref. documents No. or Test procedure No, it should be as per the current version. | |
| 6. Batch No. on all the attachments, which should be matched with COA | |
| 7. Evaluation of identification tests. | |
| 8. Attachments of the printouts, chromatograph, for the instrumental analysis like HPLC, GC, IR, UV, TLC, and Autotitrator, etc. | |
| 9. Date of analysis and the effective date for the working standard | |
| 10. The analysis completed on or before the effective date of the working standard. | |
| 11. Use of standard format for the WS COA preparation. | |
| 12. Ensure the logbook entry | |
| 13. Ensure the WS bottle label |
Prepared By : ____________ Checked By : ____________
Date : ____________ Date : ____________
Template and Relative Humidity Log of Standard’s cabinet. (Annexure 19)
Storage condition:
Month: ……………. Chamber No:__________
| Date | Time | Temperature
(in °C) |
Relative Humidity To be monitor (%) RH | Observed By | Remarks | ||
| Min. | Max | Min. | Max. | ||||
Working Standard Template issuance. (Annexure 20)
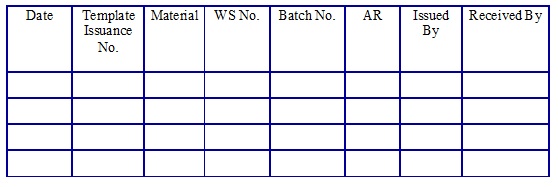
Working Standard/Working Standard Solvent Usage Log Book. (Annexure 21)
|
Standard Name |
|
| Use before date | |
| Code No. |
| Sr.No | Date of Usage | Used for
(Batch No/A.R No) |
Wt. taken
(in mg) |
Analyst sign/date | Checked by sign/date | Remarks |



Pingback: Reference Standard, Working Standard Handling - Pharma Beginners
Pingback: Reference Solution – Preparation & Hold Time Study – Pharma Beginners