 A microbial culture (microbiological culture) is a procedure of growing microbial organisms (reproduction) by allowing them to breed in programmed culture medium under controlled laboratory conditions.
A microbial culture (microbiological culture) is a procedure of growing microbial organisms (reproduction) by allowing them to breed in programmed culture medium under controlled laboratory conditions.
Microbial cultures are initial and basic diagnostic methods used as a research tool in molecular biology.
Microbial Culture Management
Standard Operating Procedure (SOP)
1.0 PURPOSE:
-
- To lay down the procedure for the management of microbial cultures.
2.0 SCOPE:
-
- This Standard Operating Procedure is applicable at Microbiology Department.
3.0 REFERENCES:
-
- Articles on Aseptic Technique for Microbiological Testing.
-
- SOP for the procurement, transfer, preservation & disposal of microbiological cultures.
-
- Disposal of used media and cultures SOP.
-
- Isolation and identification of microorganisms.
-
- USP/IP.
4.0 RESPONSIBILITY:
-
- Officer or Executive of the Microbiology Department shall be responsible for the preparation of new or revision of existing SOPs.
-
- Head of the department/designee of respective areas & QA shall be responsible for reviewing the SOPs.
-
- Plant Head and Head-Quality shall be responsible for the approval of SOP.
-
- QA shall be responsible for the distribution and control of SOPs to various departments.
5.0 ABBREVIATIONS:
-
- ATCC : American Type Culture Collection
-
- CC : Change Control
-
- °C : Degree Celsius
-
- IPA : Isopropyl alcohol
-
- LAF : Laminar Air Flow
-
- MTCC : Microbial Type Culture Collection
-
- NA : Not Applicable
-
- NCTC : National Collection of Type Cultures
-
- NCYC : National Collection Of Yeast Culture
-
- SCDA : Soya bean Casein Digest Agar
-
- SDA : Sabouraud Dextrose Agar
-
- SOP : Standard Operating Procedure
-
- v/v : Volume by Volume
6.0 PROCEDURE FOR MICROBIAL CULTURING
-
-
Procurement of Cultures:
- Prepare the list of ATCC / NCTC / NCYC / MTCC cultures required in the Microbiology section as per the details mentioned in Annexure-1.
-
-
-
- Raise the purchase requisition for the cultures for procurement.
-
-
-
- Procure the required cultures once in a year.
-
-
-
- All cultures shall be procured from the authorized sources with certificate-based on permissible subculturing periods.
-
-
-
- Ensure that the cultures shall not be more than 2 passages removed from the reference.
-
-
-
- Upon receipt of the cultures, enter the details along with in house identification no. in culture inward record as per Annexure-2.
-
-
-
- Store these cultures in the refrigerator between 2º-8ºC or as per manufacturer recommendation.
-
-
-
- For Example, E. coli received on 01/10/19 then given in house number should be E.coli 011019.
-
Also read: SOP for Isolation and Identification of Microorganisms
-
-
Reconstitution of Freeze-Dried Cultures:
- Sanitized the surface of ampoule or vial or slant or loops using 70 % IPA.
-
-
-
- Transfer the ampoule or vial or slant or loops under LAF/Biosafety cabinet and open the culture aseptically.
-
-
-
- Add 0.5 ml to 1.0 ml of sterile water to the vial/ampoule/ slant to reconstituting the lyophilized / slant cultures or reconstitute the cultures as per the recommendation of the provider.
-
-
-
- This culture will serve as mother Culture.
-
-
-
- Record the details in Culture Maintenance Record as per Annexure-3.
-
-
-
Revival and Maintenance of Cultures:
- Streak the mother culture on agar plates for confirmation of purity as per SOP for Isolation and identification of microorganisms (Annexure-4) or
-
-
-
- Direct by automated identification system and simultaneously inoculate the total content of vial/ ampoule in 100 ml of sterilized Soya bean casein digest medium.
-
-
-
- After transfer the mother culture in to the medium,
-
-
-
- Dispose the remaining content and vial as per current version of SOP for Disposal of used media and cultures. and record the details in Annexure-8.
-
-
-
- Use agar plate for purity check and
-
-
-
- Use the liquid medium for preparation of Seed lot cultures.
-
-
-
- Incubate the media containing
-
-
-
-
- Bacteria (Cultures of Bacteria) at 32.5 ± 2.5º C for 24-48 hrs,
-
-
-
-
-
- Molds (Cultures of Molds) at 22.5± 2.5º C for 72-120 hrs and
-
-
-
-
-
- Yeasts (Cultures of Yeasts) at 22.5 ± 2.5º C for 24- 48 hrs.
-
-
-
-
-
Media and incubation conditions shall be followed for different cultures as recommended in Annexure-1.
- After completion of incubation check the purity as per SOP on Isolation and identification of microorganisms of
-
-
-
-
-
- Culture by colonial characteristics,
-
-
-
-
-
- Microscopic examination,
-
-
-
-
-
- Staining and
-
-
-
-
-
- through automated identification System (BD Phoenix).
-
-
-
-
- Add 10% v/v sterile glycerol in culture suspension in 1:1 ratio, mix well and dispense 2-3 ml into the sterile cryo vial prepare 14 such vials which serves as Seed lot Culture (SLC).
-
-
-
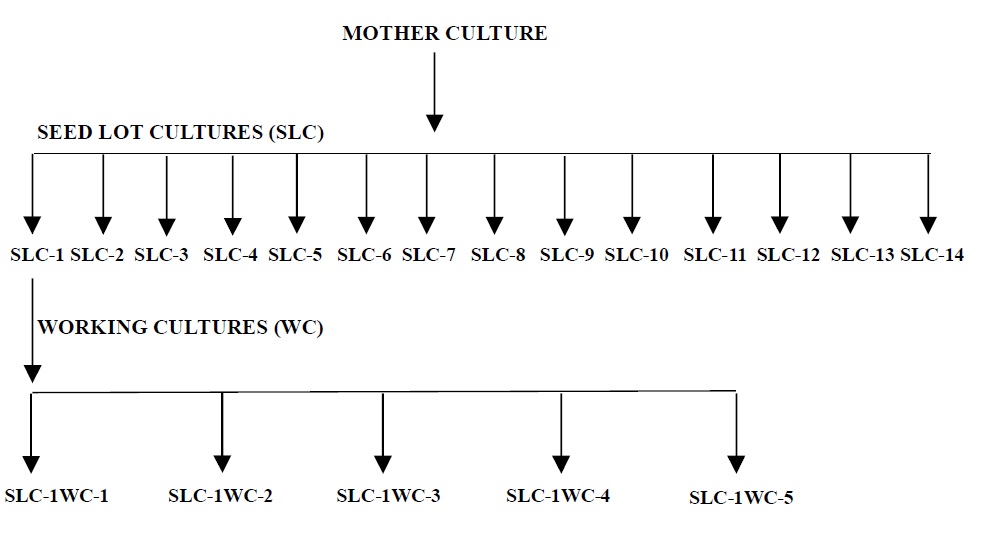
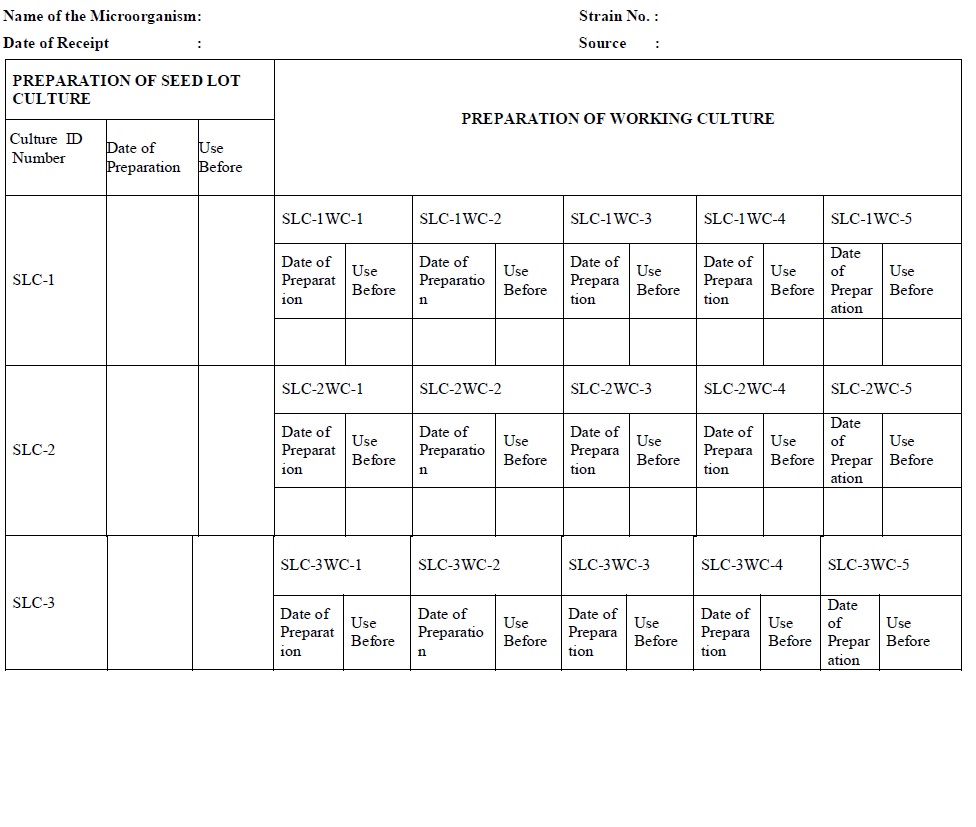
- Mark culture ID number as SLC-1, SLC-2, and SLC-3 and so on and store the cryo vials (Cryoprotective medium) at -30°C or below until use.
-
-
-
- Label each cryo vial of SLC with the details like (as per the Annexure-6.)
-
-
-
-
- Name of culture,
-
-
-
-
-
- Strain no.,
-
-
-
-
-
- Passage no.,
-
-
-
-
-
- Culture ID.
-
-
-
-
-
- Date of subculturing,
-
-
-
-
-
- Sub cultured by and
-
-
-
-
-
- Use before
-
-
-
-
- Use 12 cryovials of seed lot culture for subculturing up to 12 months (yearly) and Keep 2 cryovials as a stock which shall be used if any vial gets damaged or spillage.
-
-
-
- Ensure that the cryovials shall not be used after one year.
-
-
-
- Discard the remaining two cryovials after completion of yearly subculturing as per the current version of SOP on Disposal of used media and cultures.
-
-
-
Subculturing: (Microbial Culture)
- Maintain the cultures as per the Schematic Flow for Subculturing as per Annexure-5.
-
-
-
- For first-month subculture streak five slants of agar medium from the cryovial of SLC-1 and mark culture ID numbers as
-
-
-
-
- 1.0 SLC-1WC-1,
-
-
-
-
-
- 2.0 SLC-1WC-2,
-
-
-
-
-
- 3.0 SLC-1 WC-3,
-
-
-
-
-
- 4.0 SLC-1WC-4, and
-
-
-
-
-
- 5.0 SLC-1WC-5.
-
-
Visiters also reading :
-
-
- Simultaneously streak on the plates of agar medium for purity check as per SOP for “Isolation and identification of microorganisms” of culture by
-
-
-
-
- Colonial characteristics,
-
-
-
-
-
- Microscopic examination,
-
-
-
-
-
- Staining and
-
-
-
-
-
- through an automated identification System (BD Phoenix).
-
-
-
-
- Incubate the slants and plates containing cultures of
-
-
-
-
- Bacteria at 32.5±2.5ºC for 24-48 hrs,
-
-
-
-
-
- Molds at 22.5± 2.5ºC for 72-120 hrs. and
-
-
-
-
-
- Yeast at 22.5± 2.5ºC for 24- 48 hrs.
-
-
-
-
- When proper growth observed on the transferred slants discard SLC-1 following the current version of SOP on “Disposal of used media and cultures” and record the details in Annexure-8.
-
-
-
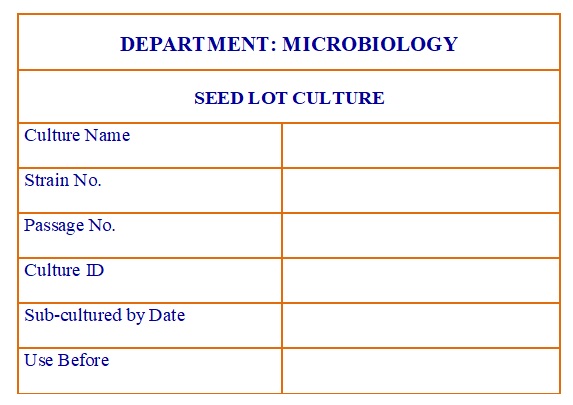
- Label each slant of working culture with the details like
-
-
-
-
- Name of culture,
-
-
-
-
-
- Strain no.,
-
-
-
-
-
- Passage no.,
-
-
-
-
-
- Seed lot culture no.,
-
-
-
-
-
- Culture ID.,
-
-
-
-
-
- Date of sub culturing,
-
-
-
-
-
- Sub cultured by and
-
-
-
-
-
- Use before as per Annexure-7 and
-
-
-
-
-
- Store the working cultures at 2 – 8 ºC.
-
-
-
-
-
- Use one working culture for each week for routine lab work for up to one month.
-
-
Discard the working culture at the end of the week or before using a new working culture.
-
-
- The fifth working culture shall also be discarded at the end of the month if it remains unused and details shall be recorded in Annexure-8.
-
-
-
- Start the same procedure with SLC-2 and so on, well before completing the cycle of previous SLC to get the working cultures ready to use for next month.
-
-
-
- Check the purity of seed lot culture and working culture as per SOP on Isolation and identification of microorganisms at the time of use by
-
-
-
-
- Colonial characteristics,
-
-
-
-
-
- Microscopic examination,
-
-
-
-
-
- Staining and
-
-
-
-
-
- Biochemical examination and
-
-
-
-
-
- Record the observations in Annexure-4 or
-
-
-
-
-
- through an automated identification system (BD Phoenix).
-
-
-
-
- Record the details of subculturing in Annexure-3 at every step of sub-culturing.
-
-
-
- Tentative Schedule for Maintenance of Microbial Cultures shall be prepared for subculturing at the time of the first revival of new cultures as per Annexure-9.
-
-
-
- Ensure that the inoculates used shall not be more than 5 passages removed from the certified reference cultures.
-
-
-
Process Description: Live Cultures
- During working with live cultures always use Gloves.
-
-
-
- Segregate all live cultures from areas used for sample testing and optimally, handled in a different area of the laboratory within a Biosafety Cabinet.
-
-
-
- Perform positive control dilutions and inoculation in a biological safety hood/cabinet.
-
-
-
- Seal Agar plates containing fungal cultures with para-film to prevent spread of spores.
-
-
-
- Surfaces in areas where a live culture plate, tube, bottle, pellet, etc., was opened shall be sanitized immediately after use by using an approved sanitizer for the appropriate contact time.
-
-
-
ANNEXURES:
- List of Microbial Cultures. (Annexure-1)
-
-
-
- Microbial Culture Inward Record. (Annexure-2)
-
-
-
- Culture Maintenance Record. (Annexure-3)
-
-
-
- Purity Check of Microbial Culture. (Annexure-4)
-
-
-
- Schematic flow for Sub-Culturing. (Annexure-5)
-
-
-
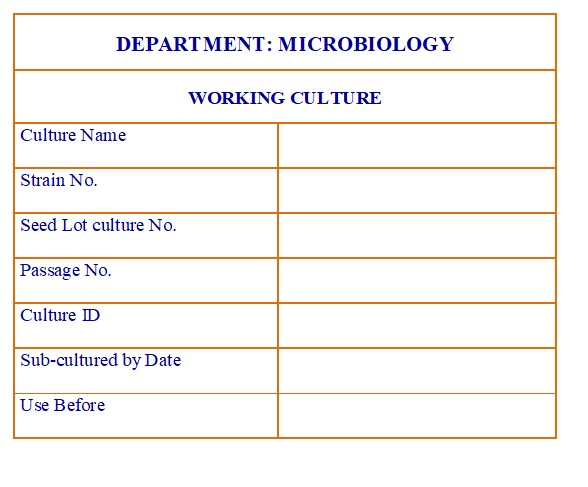
- Seed Lot Culture Label. (Annexure-6)
-
-
-
- Working Culture Label. (Annexure-7)
-
-
-
- Culture Disposal Record. (Annexure-8)
-
-
-
- Schedule for Maintenance of Microbial Cultures. (Annexure-9)
-
Click to read article : Laminar Air flow (LAF) – Operation, Cleaning and Qualification
Schematic flow for Sub-Culturing. (Annexure-5)
Seed Lot Culture Label. (Annexure-6)
Working Culture Label. (Annexure-7)
Schedule for Maintenance of Microbial Cultures. (Annexure-9)
Visiters also reading :
A) Isolation and Identification of Microorganisms
B) Microbial Culture Management



Pingback: microbiology culture procedures - infopvp
Pingback: Microscope - Operation & Calibration Procedure - Pharma Beginners
Pingback: Subculturing (Cell Passaging) in Microbiology Lab - Guidelines - SOPs