Guideline (SOP) to provide guidance for activities and analyses performed by Microbiology and Environmental Monitoring personnel in support of Aseptic Process Simulations (APS), also referred to as Media Fill Test.
Guideline for Aseptic Process Simulation (Media Fill Test)
1.0 Purpose
The purpose of this SOP is to provide guidance for activities and analyses performed by Microbiology and Environmental Monitoring personnel in support of Aseptic Process Simulations (APS), also referred to as Media Fill Test.
2.0 Scope :
This guideline includes guidance for the Microbiology laboratory and environmental monitoring department activities during initial and routine semi-annual aseptic process simulations (media fill Test) for new and existing products and processes.
Tracking and trending requirements, as well as management reporting responsibilities, are provided in order to assure management is kept apprised of any adverse trends.
The Aseptic Process Validation provides overall APS program requirements and responsibilities for all other departments. It also defines requirements for routine re-qualifications.
3.0 Responsibilities – Media Fill Test:
-
Microbiology Head / Environmental Monitoring Head shall be responsible for
-
- Assuring site procedures are in place to address all requirements in this SOP.
-
- Preparing APS protocols and summary reports along with Manufacturing and Quality Assurance.
-
- Assuring that personnel is qualified via a documented training program to collect, evaluate and test samples related to aseptic process simulations, including environmental monitoring samples.
-
- Assuring that personnel is qualified via a documented training program to test and inspect media fill Test containers including, but not limited to: growth promotion testing, an inspection of media filled containers for growth, media preparation, and microbial identification.
-
- Tracking and trending APS results and communicating this information to Quality and Operations management.
Notes:
1. Additional responsibility detail is provided in the process description and requirements sections of this SOP for Media Fill Test.
2. Responsibilities in this SOP are suggested based on Workflow (Media Fill Test).
4.0 Definitions and Abbreviations – Media Fill Test:
| Aseptic | Absence of viable microorganisms |
| Aseptic Filling | Part of aseptic processing in which a pre-sterilized product is filled and/or packaged into sterile or depyrogenated containers and partially closed and/or closed |
| Aseptic Process Simulation (APS) | A means for establishing the capability of an aseptic process as performed using a growth medium in place of the typically filled material. Note that APS is understood to be synonymous with media fill Test. |
| Aseptic Processing | Assembly of sterilized components and products in a controlled environment, in which the air supply, materials, equipment, and personnel are regulated to control microbial and particulate contamination to acceptable levels |
| Aseptic Processing Area | An area that has defined environmental control of particulate and microbial contamination, and is constructed and used in such a way as to reduce the introduction, generation, and retention of contaminants within the area used for processing of sterile products |
Growth Promotion Test (GP or GPT) |
Test performed to demonstrate that media will support microbial growth, as required by Pharmacopeia that specifies challenge organisms, inoculum level, and incubation conditions |
| Intervention | An aseptic manipulation or activity performed by personnel that occurs within the critical area |
| Intervention, Corrective (Non-Routine) | An intervention is performed to correct or adjust an aseptic process during its execution. Examples include: clearing component misfeeds, adjusting sensors, and replacing equipment components |
| Intervention, Inherent (Routine) | An intervention that is an integral part of the aseptic process and is required for set-up or routine operation and/or monitoring, e.g., aseptic assembly, container replenishment, environmental sampling, etc. inherent interventions are required by the batch record, procedure, or work instruction for the proper conduct of the aseptic process |
| Media Fill Test | Refer to Aseptic Process Simulation |
| Sanitization | The act or process, physical or chemical, of reducing viable organisms on a surface to a defined acceptable level |
| Sterile | Absence of any viable organisms |
| Sterility Test | Tests performed to determine if viable microorganisms are present |
| Sterilization | The act or process, physical or chemical, of destruction or elimination of all viable organisms |
5.0 Work Flow – Media Fill Test:
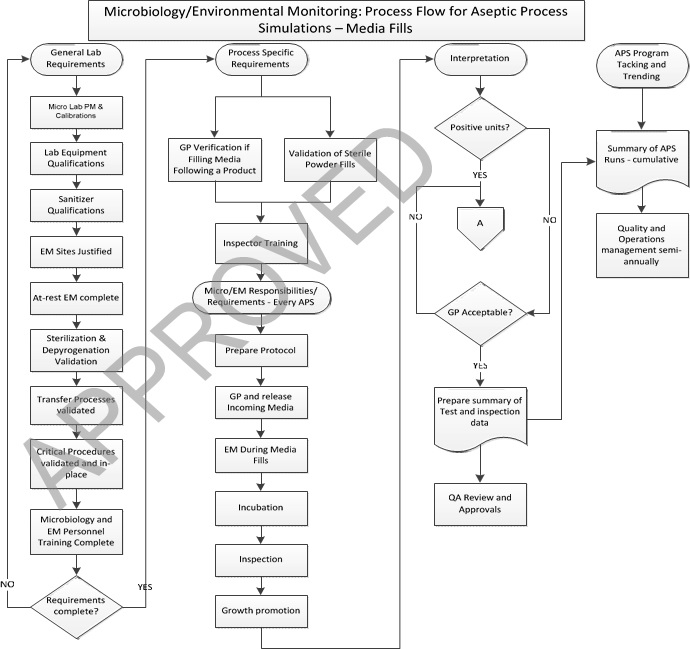
6.0 Procedure – Microbiology Laboratory Procedures For Aseptic Process Simulations (Media Fill Test)
-
General microbiology and environmental monitoring laboratory minimum requirements prior to initiating aseptic process simulations (APS/Media Fill Test) for a new line:
- Preventive Maintenance Program (PM) shall be in place for incubators, environmental monitoring test equipment, and laboratory instrumentation used during Media Fill Test studies, and all PMs must be current prior to using any equipment and instrument.
-
- Calibration programs shall be in place for incubators, environmental monitoring test equipment, and laboratory instrumentation used for Media Fill Test studies and all calibrations must be current prior to using any equipment and instrument.
-
-
All equipment, including, but not limited to,
-
-
-
- Incubators sterilizers,
-
-
-
- Laminar flow hoods,
-
-
-
- Isolators,
-
-
-
- Water baths, and
-
-
-
- Environmental monitoring devices such as particle counters and microbial air samplers have been qualified and summary reports are reviewed and approved by QA (refer to the SOP for Environmental Monitoring Program).
-
-
- Monitoring systems for incubators have been qualified and summary reports are reviewed and approved by Quality Head.
-
- Sanitizer qualification studies have been completed for all surfaces in the aseptic processing area, and the summary report is reviewed and approved by Quality Head.
-
- Environmental monitoring sites, including personnel gowns and gloves, have been selected and their fitness of use justified. Initial at- rest environmental monitoring qualification has been completed for the line/area and summary reports are reviewed and approved by QA.
-
- Sterilization and depyrogenation processes for all microbiological sample and test equipment, media, and environmental test equipment, have been validated and summary reports are reviewed and approved by QA
-
- Transfer of sterilized sample equipment to the aseptic processing area and lines has been validated to prevent contamination of the equipment prior to use.
-
-
Validated procedures are in place for, but not limited to:
- Environmental monitoring.
-
-
-
- Cleaning and sanitization of aseptic test areas and equipment in microbiology and environmental monitoring laboratories.
-
-
-
- Media filled unit growth promotion
-
-
-
- Quality testing of incoming media prior to use in Media Fill Test studies.
-
-
-
- Preparation of media
-
-
-
- Media Fill Test incubation
-
-
-
- Inspection of media filled units
-
-
-
- Reporting Media fill test results
-
-
-
- Tracking and trending Media fill test results
-
-
-
- Reconciliation of media-filled units.
-
-
- Microbiology and environmental monitoring personnel have been adequately trained and qualified to the procedures listed above, and written documentation of this training is available and current.
-
- Media fill test inspection training and qualification have been completed for personnel assigned to media fill test/inspection.
-
- Microbiology and environmental monitoring personnel assigned to perform activities during the media runs must be properly trained on the requirement in the media run protocol as well as the tasks to be performed.
-
- Microbiology and environmental monitoring personnel entering aseptic processing areas must be trained and qualified to enter the area.
-
Process specific microbiological test and method requirements before running aseptic process simulations:
-
-
Verification of growth promotion in media filled in equipment immediately after product:
-
-
- If media is to be filled directly after a product run without changing equipment or parts in the fluid path, a protocol must be prepared to validate the effectiveness of the fluid path flush to eliminate any growth inhibition in media filled after the flush.
-
- Studies shall also confirm that there is no interaction between product and media that could produce cloudiness, precipitate, or other material that could interfere with the detection of growth during the inspection.
-
- Pharmacopeia specified growth promotion organisms and representative in-house environmental organisms shall be used to confirm media growth capability.
-
- Repeat protocol studies at least in triplicate to verify the flush is reproducible.
-
Validation of sterile powders (Media Fill Test – Dry Powder Injection):
-
- Sterile powder fills or simulation of sterile suspensions requires the use of sterilized powders, such as Lactose, that will not inhibit the growth of organisms and will not interfere with the ability to detect growth during the inspection.
-
- Sterilization of the powder shall be validated.
-
- The sterility of the placebo powder shall be verified as per the validated sterility test method prior to use in a Media Fill Test.
-
- Prepare a protocol to perform growth promotion testing of solutions made with the placebo powder and media at concentrations to be used in APS studies.
-
- The protocol shall also contain verification that the powder is soluble and the resulting solution of powder and media does not contain any particulate matter or cloudiness that would interfere with the detection of growth during the Media Fill Test.
-
Training operators for media-filled unit inspection:
-
- The inspection shall be done by qualified microbiologists or personnel trained by qualified microbiologists to recognize contaminated media-filled containers.
-
- Training shall include examples of contaminated media-filled containers.
-
- Inspectors shall demonstrate the consistent ability to detect low turbidity contamination.
-
- Document all training exercises in the employee training file.
-
- If clear, non-colored containers are not available for an APS, each container must be aseptically transferred to a clear container for inspection after incubation is completed.
-
- Inspectors must have documented training for the process of transferring and evaluation of the sample in the final clear container. Low and high turbidity contamination shall be included in this training.
-
- Inspectors shall be trained to assure that units are maintained in their original tray. There shall be no back-filling with units from other trays to assure that the fill order remains traceable
-
Microbiology and environmental monitoring requirements for every Media Fill Test:
-
-
Protocol Preparation:
-
-
- Protocols shall contain all requirements as per this SOP and as per the SOP – Aseptic Process Validation. Microbiology and environmental responsibilities shall be specified in the protocol.
-
- Protocols shall be approved prior to execution.
-
- Changes/modifications to the protocol after approval, if any, shall be routed through Change Control, and revision of the Protocol shall be approved before execution (refer to SOP: Change Control Management).
- The same protocol shall be used if there is no change from the previous qualifications.
- Media growth promotion prior to use in APS studies/Verification of the non-inhibitory property of the powders used for simulation:
- As part of incoming materials quality verification, perform growth promotion testing of media/powder received/used or manufactured in-house for use in APS studies.
-
- Use Pharmacopeia specified growth promotion organisms as well as representative organisms found during environmental monitoring.
-
- Also challenge with any organisms from sterility test positives, if applicable.
-
- Sterile powders, such as Lactose for use in media fills, shall be sterility tested and confirmed to be non-inhibitory by performing growth promotion on a media/sterile powder solution at the concentration to be used in Media Fill Test/studies.
-
- Media shall be released for use only after successful growth promotion testing.
-
- Sterile powder shall be released for use only after successful sterility testing and successful growth promotion in media/powder solution.
-
Environmental Monitoring during Media Fill Test:
- Environmental monitoring shall be performed throughout set-up and during the entire Media Fill Test, at all sample sites monitored during routine production runs.
-
- Contact plates for gloves of operators shall be sampled after all corrective interventions and upon every exit from the area.
-
Incubation requirements for filled units used for API Process Simulation assurance:
- Incubate filled units in qualified incubators monitored by qualified and calibrated temperature monitoring systems.
-
- Units may be incubated upright after they have been inverted to wet al internal surfaces with media. Media may also be incubated inverted.
-
- Incubate filled units at 20°C-25°C for seven (7) days and then transferred to second incubator at 30°C-35°C for seven (7) days; there shall be a total minimum of fourteen (14) days of incubation.
-
- The incubation temperature shall be monitored continuously and recorded throughout the duration.
-
- If temperature excursions occur, open an investigation and determine impact on media within the incubator and corrective actions that may include extending incubation time.
-
Inspection requirements during Media Fill Test:
- Inspection shall be done by qualified microbiologists or personnel trained by qualified microbiologists to recognize contaminated media filled containers.
-
- Random checking shall be done by qualified microbiologists.
-
- Filled container inspection shall be performed prior to placing into the incubator in order to remove obvious nonintegral units (e.g. misaligned closures, cracks in glass, missing or leaking crimps).
-
- For the purposes of Media Fill Test, cosmetic, particulate and fill volume defects shall be ignored, incubated and the results included in the APS evaluation and contamination rate.
-
- Unit accountability and reconciliation shall be maintained and documented before and after each inspection period.
-
- Opaque, non-clear, or dark colored containers shall be inspected only after the full 14 day incubation period because the contents require transfer into clear containers for inspection.
-
- Inspection shall be performed after seven (7) days of incubation at 20°C-25°C, prior to transferring into the 30°C- 35°C incubator.
-
- Final inspection shall be performed after at least seven (7) days of incubation at 30°C-35°C.
-
- Any unit with suspected growth shall be segregated, its location within the batch documented, and examined by a trained Microbiologist.
-
- Suspected contaminated units shall be sub-cultured upon discovery and results reported.
-
- If container / closure defects are detected during post incubation inspection, the root cause of the defect must be investigated with a corrective action.
-
Growth promotion testing during Media Fill Test:
- 7.3.6.1 After the fourteen (14) day incubation period, growth promotion (GP) shall be performed on samples from the beginning and end of the run.
- Sufficient filled media containers should be sampled from the beginning and end of each APS to perform growth promotion of all organisms on each set.
-
- Maintain traceability of the samples.
-
- Growth promotion testing shall be performed in duplicate (a beginning and an end sample set) after the fourteen (14) day incubation using organisms, inocula counts and incubation conditions listed in USP <71>, as well as representative organisms found during environmental monitoring. Also challenge with any organisms from sterility test positives, if applicable.
-
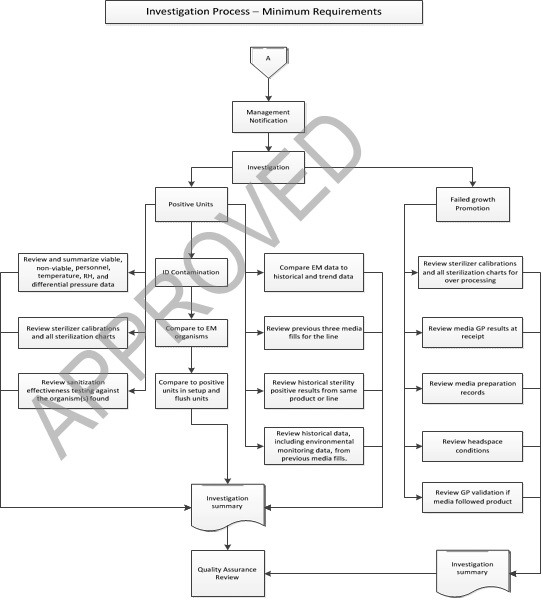
Interpretation of Results and Investigations of Media Fill Test:
- Any positive unit/presence of growth shall result in a thorough, documented investigation.
-
- Following the investigation, appropriate corrective action may be taken based on scientific evaluation and risk assessment (refer to the SOP : Investigations and SOP : Handling of Corrective and Preventive Actions (CAPA)).
-
- Quality and Operations management shall be notified within 1 business day of confirmation of positive units.
-
-
Minimum investigation by microbiology/environmental Monitoring should include
-
-
-
- Positive Media filled Unit Microbiology and Environmental Investigation Considerations.
-
-
-
- Include the identification to genus, and species, if possible, of any microorganisms found on environmental monitoring samples or in media fill containers.
-
-
-
- Compare organisms found in container with any found in the environment or on personnel.
-
-
-
- Review setup and flush for positive units in conjunction with the investigation.
-
-
-
- Review and summarize all environmental monitoring data associated with the media compounding and/or filling process, including areas designated for storage of components.
-
-
-
-
This includes viable, non-viable, personnel, temperature, RH, and differential pressures.
-
-
-
-
- Review the historical and trend data for a minimum of ten previous environmental monitoring sessions for both the room where the Media Fill Test occurred, and the remainder of the aseptic area. Compare the results from the Media Fill Test with the trend data from product fills.
-
-
-
- Discuss and Review the historical data, including environmental monitoring data, from previous APSs. Minimally review and discuss the previous three APSs for the same line.
-
-
-
- Review and discuss the historical sterility positive results from the same product or filling line since the last successful media simulation.
-
-
-
- Review calibration status of sterilizers and all sterilization charts for media, components and equipment from the Media Fill Test, for adherence to specifications, and discuss.
-
-
-
- Discuss and Review Media Fill Test results of any available sanitization effectiveness testing against the organism(s) found.
-
-
-
- Review and discuss container closure integrity test data.
-
-
- All positive units shall be identified to at least the genus, and to the species level using genomic methods, where possible.
-
- A comprehensive sampling and identification scheme is critical in the investigation and determination of the contaminant source.
-
- When positive units are encountered, all possible sources of contamination shall be investigated.
-
- A detailed history of the investigation shall be maintained.
-
- The identification of the contaminating organism shall be compared to the database of the organisms identified within the facility through the environmental monitoring program.
-
- Biochemical and/ or genetic profile of the contaminating microorganisms shall also be compared to that of microorganisms obtained from testing programs including sterility tests, bio burden and environmental monitoring programs (air viable, equipment surfaces, water systems and personnel), in order to help identify the potential sources of the contaminant.
-
An Investigation report shall be prepared and, at a minimum, include the following:
- An overall summary of what was reviewed during the investigation.
-
- A table summarizing the source documentation (including Environmental Monitoring data) and the results of each of the findings.
-
- A historical time sequence of activities leading up to the failed unit(s).
-
- Probable root cause of the positive (failed) unit(s) and a conclusion from the findings.
-
- An impact assessment that considers previously executed Media Fill Test up to the APS batch with the positive (failed) unit(s).
-
- The CAPA plan (immediate and longer term actions).
-
- If growth promotion fails, open an investigation to determine cause.
-
-
The investigation shall minimally include:
-
-
- Review sterilizer calibrations and all sterilization charts for evidence of over processing, if media was heat sterilized.
-
- If media was filter sterilized, review the filter integrity test results and any observations of the filter after use that may suggest filter plugging from undissolved media.
-
- Review raw media GP results from acceptance testing at receipt.
-
- Media preparation records Review to assure that media was properly formulated, dissolved and filtered.
-
- Review target headspace volume to assure sufficient space to maintain aerobic conditions. Typically volume is half filled or less, but sufficient to allow media contact with all internal surfaces when inverted. Growth of only anaerobic challenges could indicate insufficient headspace oxygen.
-
- Review GP validation, if media was filled immediately after product, and compare them against the actual media filling conditions.
-
- Provide a CAPA plan to correct issues before repeating the run.
-
Media Fill Test Program Tracking and Trending :
- Microbiology or Environmental monitoring shall maintain a cumulative summary of all aseptic process simulations, including initial studies.
-
- This summary shall be updated after each new APS is complete. The summary shall include a table with the following information, at a minimum:
-
-
- Line #
-
-
-
- Process/product being simulated
-
-
-
- Date of Media Fill Test
-
-
-
- Container used
-
-
-
- Number of units filled and incubated
-
-
-
- Number of contaminated units
-
-
-
- Length of the run
-
-
-
- Type of run (routine, initial validation, change control, etc.)
-
-
-
- The summary shall be circulated to Quality and Operations management at least semi-annually.
-
-
Media Fill Test Requirements:
- Minimum APS Related Requirements for Microbiology and Environmental Monitoring Laboratories/Groups:
-
- Each site shall have a written program for routine aseptic process simulations.
-
- Site procedures shall be developed and in place for all Microbiological and Environmental monitoring sampling and testing processes required to support APS studies, including:
-
-
- Environmental monitoring
-
-
-
- Cleaning and sanitization of aseptic test areas
and equipment in laboratories.
- Cleaning and sanitization of aseptic test areas
-
-
-
- Sterilization of sample and test utensils, equipment and apparatus.
-
-
-
- Acceptable transfer of sterilized sample equipment to aseptic processing areas in manufacturing and laboratories.
-
-
-
- Media growth promotion, including handling failing GPT results.
-
-
-
- Quality testing of incoming media prior to use.
-
-
-
- Preparation of media
-
-
-
- Media fill incubation Test
-
-
-
- Inspection and interpretation of media filled units showing growth.
-
-
-
- Microorganism identification.
-
-
-
- Investigation process for media filled units showing growth.
-
-
-
- Tracking, trending and reporting cumulative Media Fill Test results semi-annually.
-
-
-
- Reconciliation of media filled units.
-
-
- Protocols with Microbiology and Environmental Monitoring requirements and department responsibilities shall be required for all Media Fill Test studies.
-
- Preventive Maintenance and Calibration Programs shall be in place for all Microbiology and Environmental Monitoring equipment and instruments.
-
-
Qualification of all Microbiology and Environmental Monitoring equipment shall have been reviewed and approved by QA.
-
-
- Procedures shall be in place for performing sanitizer qualification studies.
-
- Sterilization and depyrogenating processes for all microbiological sample and test equipment, media and environmental test equipment, have been validated and operation procedures in place.
-
- Microbiology and environmental monitoring personnel have been adequately trained and qualified to the procedures listed above, and written documentation of this training is available and current.
-
- Personnel assigned to perform activities during the media runs must be properly trained on the requirement in the media run protocol as well as the tasks to be performed.
-
- Microbiology and environmental monitoring personnel entering aseptic processing areas must be trained and qualified to enter the area.
7.0 REFERENCES
-
- Pharmaceutical Inspection Convention, Pharmaceutical Inspection Co-Operation Scheme (PIC/S), Recommendation on the Validation of Aseptic Processes (PI 007-6).
-
- FDA Guidance for Industry – Sterile Drug Products Produced by Aseptic Processing – Current Good Manufacturing Practice.
-
- Parenteral Drug Association Technical Report No. 13 (Revised), Fundamentals of an Environmental Monitoring Program.
-
- Technical Report No. 22 (Revised 2011), Process Simulation for Aseptically Filled Products.
-
- Parenteral Drug Association Technical Report No. 44, Quality Risk Management for Aseptic Processes, Vol. 62, No. S-1 2008.
-
- Parenteral Drug Association Technical Report No. 28, Process Simulation Testing for Sterile Bulk Pharmaceutical Chemicals, Vol. 60, No. S-2, 2006.
-
- USP <1116> Microbiological Control and Monitoring of Aseptic Processing Environments.
-
- Volume 4, EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, Annex 1 Manufacture of Sterile Medicinal
Products USP <71> Sterility tests.
- Volume 4, EU Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use, Annex 1 Manufacture of Sterile Medicinal
****************** To get this doc : write us- [email protected] **************************


