Standard Operating Procedure (SOP) for preparation, Issuance, and Execution of Stability Study Protocol, Analytical Template, and Specification of Drug Product.
Stability Study Protocol, Template & Specification Preparation
1.0 PURPOSE:
-
- The purpose of this Standard Operating Procedure (SOP) is to describe the procedure for the preparation of the stability study protocol/template/Specification in the Quality Control department.
2.0 SCOPE – SOP FOR STABILITY STUDY PROTOCOL:
-
- This SOP is applicable for the preparation of stability protocol for Drug products packed in the proposed containers and closures for marketing, Template, and Specification which are manufactured in the quality control department.
3.0 REFERENCES:
-
- ICH Guideline on “Stability Testing of new drug substances and products Q1A (R2) and ICH-Q1B”
4.0 RESPONSIBILITY:

-
-
QC Officer/Executive :
-
-
- To prepare the stability protocol as per the SOP.
-
- To record the raw data as per SOP.
-
-
Quality Control Head or Designee :
-
-
- To provide the training to the concern before the implementation of SOP.
-
- To ensure the availability of the stability-indicating method.
-
-
Quality Assurance :
-
-
- To ensure the implementation of the system as per SOP.
-
-
Quality Head and Plant Head :
-
-
- To review and approve the SOP.
5.0 ABBREVIATIONS USED IN SOP FOR STABILITY STUDY PROTOCOL:
-
- ACC: Accelerated Condition
-
- ICH: International Conference On Harmonisation
-
- LIMS: Laboratory Information Management System
-
- LTC : Long Term Condition
6.0 PROCEDURE – SOP FOR STABILITY STUDY PROTOCOL:
- General Procedure :
-
- Head QC or Designee shall ensure the availability of the stability-indicating method.
-
- Generally, the finish product release testing procedure is a stability-indicating method so, the same standard method is given in “Stability Study Protocol/template/specification”.
-
- If the stability-indicating the method is different then the finished product then separates standard method is given in “Stability Study Protocol/template/specification”.
-
- If the product is as per any Pharmacopoeia and stability testing method is not available, then consider the Pharmacopoeia as reference for the preparation of stability Study Protocol/Template/Specification.
-
- “Stability Study protocol” can be prepared as per the requirement of the regulated agencies & product characteristic, on the basis of the Stability program SOP.
-
- Whenever bracketing and matrixing are applicable, stability study shall design accordingly.
-
- The orientation of the storage of the drug product to be decided as per the attached stability analysis test guideline.
-
- Mark the stability station time points with (ü ) wherever applicable.
-
Stability Study Protocol Preparation :
-
- The stability Study protocol number shall be given as.
SP/001
SP = Stand for Stability Study Protocol
001= Stand for the Serial No.of Stability Study Protocol
(Example: First protocol No.shall be SP/001 and so on.)
-
- Revise Stability Study protocol number shall be given as.
SP/001/01
01 = Stand for first revision No. of Protocol
(Example: First revision No. of the protocol shall be SP/001/01 and so on.)
-
- If editorial changes are applicable to stability, the Stability Study Protocol number shall be given as.
SP/001/E1
E1 = Stand for Editorial changes E1
-
- Stability Study protocol shall be prepared by stability Section Head, checked by the designated person, and approved by Head Quality or designee.
-
- Stability Study protocol shall be designed in such a way, that it provides information about the..
-
-
- Purpose
-
-
-
- Scope
-
-
-
- Annexure (Accelerated Condition and Long Term Condition have information about Stability data, Purpose for stability, Label claim, Stability Summary Report, and Conclusion)
-
-
-
- Procedure (Stability Conditions (Accelerated Condition and Long Term Condition),
-
-
-
- Criteria for stability study of selection batch,
-
-
-
- Stability Study Programme(Stability sample and testing time points for chemical analysis, Stability sample and testing time points for Microbiological analysis, Chemical stability test analysis and Microbiological stability test analysis) and
-
-
-
- History log.
-
-
-
Stability station for ACC, Long term stability condition shall be as follows.
- For: Accelerated condition : 0M, 3M, 6M
-
-
-
- For: Long term stability condition: 0M, 3M, 6M, 9M, 12M, 18M, 24M, 36M, 48M, 60M up to the shelf life of the product and additional 12 months thereafter for the extension of expiration dating as per the requirement.
-
-
- Tests required for the stability study shall be respective to the type of dosage form and shall be indicated as per the “stability study test guideline” (Annexure-3).
-
- Test which has a “#” mark in the “stability analysis test guideline” shall apply for that dosage form and shall be selected for the test and for the evaluation parameters.
-
- The sample quantity to be taken shall be decided base on the final product test procedure for each stability station and shall be taken 1.5 times excess for the evaluation purpose or as specified in the Stability Study protocol.
-
- Protocol for Stability Study shall contain the “Stability analytical specification”, which gives information about the tests to be carried out and the evaluation parameter.
-
Stability Template Preparation in LIMS :
-
- Stability Analytical Template shall be prepared by the LIMS Department and send to Section Head at the plant for checking and at the time of preparation mention all changes in the History log.
-
- After checking LIMS Department sends to CQ for final Approval of template through LIMS Software.
-
- Approval of the template, select the test plan and then send for checking to Section Head at the plant.
-
- After checking, Head Quality Control and Head Regulatory approved the template at the plant through LIMS Software and After approval of template, Section Head can be used the template for routine analysis.
-
-
Analytical Template shall be designed in such a way that it provides information about the…
-
-
-
- Product
-
-
-
- Generic Name
-
-
-
- Specification No.
-
-
-
- Revision No.
-
-
-
- Batch No.
-
-
-
- Batch Size
-
-
-
- Mfg. Date
-
-
-
- Exp. Date
-
-
-
- Change Control No. (SOP for Change Control Management)
-
-
-
- Effective Date
-
-
-
- Sample ID
-
-
-
- Qty. sampled
-
-
-
- Date of Release
-
-
-
- Date of Analysis
-
-
-
- Type of Tests
-
-
-
- Sr.No. of test
-
-
-
- Specification limit
-
-
-
- Test procedure
-
-
-
- Observations
-
-
-
- Analysed By/Date
-
-
-
- Checked By/Date
-
-
-
- Prepared By
-
-
-
- Approved By and
-
-
-
- Details of leftover sample”.
-
-
- While auto-generated “Stability Study Protocol No”, “Storage condition”, “Packing style”, “Orientation”.
-
- Assign the Template No. in Template Issuance Register as per respective SOP.
-
-
Template Number allotted for the generation of worksheet during registration as follows.
-
STB/ZZ/YMACC/XXXX
STB/ZZ/YMLTC/XXXX
STB = Stand for Stability
ZZ = Last two digit of year
YM = Stand for 1 Month, 2Month…
ACC = Accelerated Climatic Condition
LTC = Long Term Condition
XXXX = stand for Serial No. 0001, 0002,……,
-
- Generate the Analytical template through LIMS after assigning the AR.No. of respective products for analysis.
-
Stability Specification Preparation :
-
- Procedure for Preparation of Analytical Template and Specification
Step: 1
LIMS Department Analytical Template and Specification
Step: 2
Section Head shall check the Analytical Template and Specification.
Step: 3
CQ shall Approve the Analytical Template and Specification.
Step: 4
After CQ Approval, the LIMS department shall select the test plan.
Step: 5
After selection of test plan Section Head shall check once again Analytical Template and Specification.
Step: 6
Finally Quality Head and Regulatory Affairs shall approve Analytical Template and Specification at the plant.
7.0 ANNEXURES – SOP FOR STABILITY STUDY PROTOCOL:
Annexure 1: Stability Study Protocol
| STABILITY STUDY PROTOCOL | Page X of Y | |||
| Product | ||||
| Generic Name | ||||
| Label Claim | ||||
| Protocol # : | CC # : | Revision No :
Effective Date : |
||
| Prepared By | Checked By | Approved By |
- Purpose : __________________________________________________
- Scope : ___________________________________________________
- Attachment’s (s) : _____________________________________________
- Procedure :
-
Stability Conditions :
-
-
- At Accelerated (0,3 & 6 Month), Temperature : 40±2°C and 75±5% RH
-
- At Long term (36 Month), Temperature as specified in the protocol.
-
-
Criteria for stability study of selection batch :
-
-
- Manufacturing formula.
-
- Manufacturing procedure.
-
- Major equipment change.
-
- Change in Primary packing material.
-
- Yearly stability of the product
-
- New vendor of Raw material / Packing material.
-
-
Study Programme :
-
-
- Stability sample and testing time points for chemical analysis
|
Stability Type / Condition |
||||
|
ACC: 40±2°C and 75±5% RH |
Sample Qty(Tabs) | LTC * |
Sample Qty(Tabs) |
|
| Stability Station(Month) | ||||
| 0M | ||||
| 3M | ||||
| 6M | ||||
| 9M | ||||
| 12M | ||||
| 18M | ||||
| 24M | ||||
| 36M | ||||
| Stability Station (Month) | ||||
| No. of Stability Station | ||||
| Total qty. /Station | ||||
| Container / Sample qty. to drawn (1.5 times) or * | ||||
-
- Stability Sample and testing time points for Microbiological analysis
|
Type / Condition |
||||
| ACC: 40±2°C and75±5% RH | Sample Qty(Tabs) | LTC* | Sample Qty(Tabs) | |
| Stability Station (Month) Or * | ||||
| 0M | ||||
| 3M | ||||
| 6M | ||||
| 9M | ||||
| 12M | ||||
| 18M | ||||
| 24M | ||||
| 36M | ||||
| No. of Stability Station | ||||
| Total qty. /Station Or * | ||||
-
- Chemical Stability test analysis :
| Sr. | Test | Specification | Test-Method |
- HISTORY LOG
| Revision No | Effective Date | Supersede | Supersede Date | Main Spec Rev. No. | CC No |
*As per Specified protocol
Annexure 2: Stability Study Analytical Template
For : Stability Study
| Product | : | ||||
| Generic Name | : | ||||
| Specification No. | : | CC No. | : | ||
| Revision No. | : | Effective Date | : | ||
| Batch No. | : | Sample No | : | ||
| Batch Size | : | Qty. Sampled | : | ||
| Mfg. date | : | Sampled By/Date | : | ||
| Exp. Date | : | Date of Release | : | ||
| Prepared By | Checked By |
| Approved By | |
| Head Quality | Regulatory Affairs |
Stability Analytical Template shall contain following details but not limited to :
-
- Description :
-
- Identification :
-
- Average weight :
-
- Friability/ Disintegration :
-
- Dissolution :
-
- Degradation Product / Related Substance :
-
- Assay :
-
- Microbial test :
| Analyzed By : | Date : |
| Checked By : | Date : |
Checker Remarks :
Left over sample has been destroyed after analysis.
Sample Quantity:_____________ By:________ Date :_______
HISTORY LOG
| Revision No | Effective Date | Supersede | Supersede Date | Main Spec Rev. No. | CC No |
Annexure 3: Stability Analysis Test Guideline
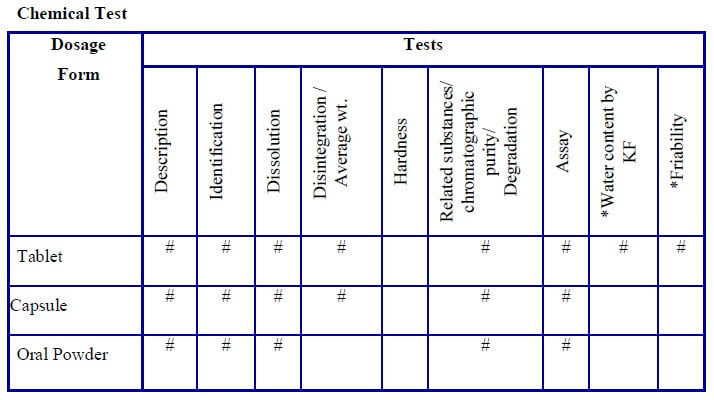
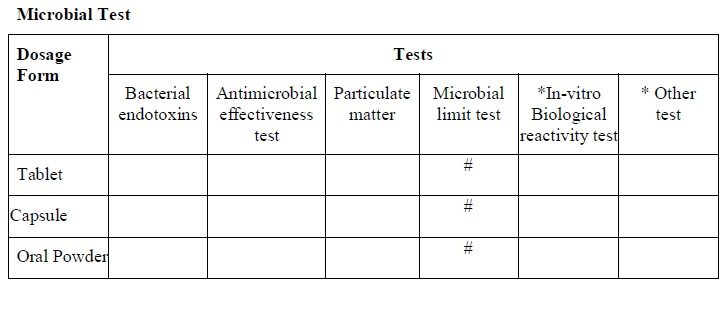
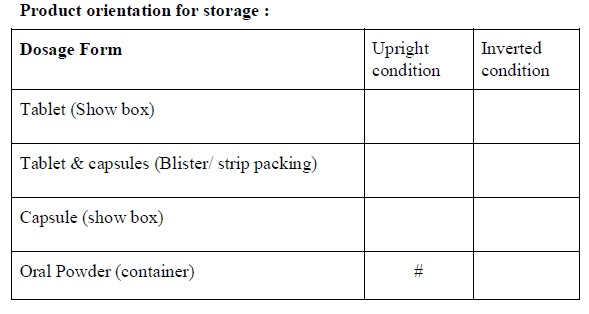
* If applicable
# Test performed
-
- All of the above tests/parameters are not mandatory.
-
- Tests/parameters can differ based on the type of formulation, product nature, and the final product specification
Annexure 4: Stability Study Analytical Specification
For: Stability Studies
| Product :
Generic Name : Label Claim : |
Page X of Y | |||
| Code No : | Spec No : | Rev. No : | CC No: | Effective Date : |
| Prepared By | Checked By |
| Approved By | |
| Head Quality Control | Head Regulatory |
| Reference : | ||||
| Pharmacopeial Status | ||||
| Page No. | ||||
| Reviewed By | ||||
| Effective Date | ||||
| Sr. | Test | Specification | Method-Test |
Annexure 5: Stability Study Protocol Index
| Sr.No. | Product Name | Protocol No. | Revision No. | Effective Date |


