Reduce Testing in Raw Material
Standard Operating Procedure (SOP) for Reduce Testing in Raw Material (API and excipients), This will help to increase the productivity of Quality Control for speedy release of RM.
1.0 PURPOSE:
-
- The purpose of this SOP is to describe the procedure for reduce testing in analysis of raw materials..
2.0 SCOPE:
-
- This procedure is applicable to the analysis of raw materials consignments (drug substances and excipients.) received from the approved vendor.
3.0 REFERENCES:
-
- In-house.
-
- Sampling procedure of raw material SOP.
-
- SOP for Receipt, testing and release of in-process, finished product and miscellaneous items.
-
- Investigation of Out of Specification Analysis Result SOP.
4.0 RESPONSIBILITY:
-
-
QC Personnel shall be responsible for:
- To check integrity of the consignment at the time of sampling.
-
-
- To check consignment for its identity, intactness and physical condition.
-
- Ensure approved label of supplier on container.
-
- To check the compliance of the COA with the specification.
-
- Sample the consignment as per general sampling practices given in the SOP of “Sampling procedure of raw materials”.
-
- Prepare reduce testing proposal report.
-
Quality Control Head or Designee shall be responsible for:
- Ensure implementation and adherence to the system as per the SOP.
-
- Review reduce testing proposal report.
-
- Communicate discrepancies to the corporate compliance and purchase department.
-
- Evaluate the impact of the material’s failure with respect to test in co-ordination with FDD, ADD and CQ.
-
-
Quality Assurance shall be responsible for:
- To check the SOP.
-
-
- To ensure implementation of the system as per the SOP.
-
-
Site Quality Head shall be responsible for :
- To review and approve the SOP.
-
-
- To ensure implementation of the system as per the SOP.
5.0 ABBREVIATION:
-
- ADD : Analytical Development Department
-
- COA : Certificate of Analysis.
-
- CQ : Corporate Quality
-
- DMF : Drug Master File.
-
- FDD : Formulation Development Department
-
- IR : Infrared
-
- LIMS : Laboratory Information Management System
-
- LOD : Loss on Drying
-
- NIR : Near Infrared
-
- QA : Quality Assurance
-
- QC : Quality Control
-
- SOP : Standard operating procedure.
6.0 DEFINITION:
-
-
Approved Vendor :
- A vendor which has been qualified as per the vendor qualification SOP, irrespective of whether the vendor is DMF holder or not.
-
-
-
LOD :
- Loss on drying is the Loss of weight expressed as percentage w/w resulting from water and volatile matter of any kind that can be driven off under specified condition.
-
7.0 PROCEDURE:
-
-
- Prior to start up of reduce testing, the designed person from QC (i.e Section in-charge, officer, analyst etc.) shall prepare the reduce testing proposal report and submit the same to QA
-
-
-
- Assign the report number as follows :
-
RED/X/YY/ZZZ,
Where,
RED stands for Reduce Testing,
X stands for name of the material .
YY stand for current year and
ZZZ stand for serial No. start from 001, and so on.
-
-
- Head Quality or designee from QA shall approve the proposal.
-
-
-
- Vendor Identification for reduce testing.
-
-
-
- When the material is received from new vendor then the first five consignments is to be analyzed for complete analysis.
-
-
-
- If all the results are found satisfactory then reduce testing is to be followed for the same.
-
-
-
- If the material is received from an approved vendor but less than five consignments from the vendor have been analyzed for complete analysis.
-
-
-
- Then the material from the vendor shall be considered for reduce testing only.
-
-
-
- When the complete analysis of five consignments complies satisfactorily as per the specification.
-
-
-
Prepare a risk assessment report and consider the following data ..
-
-
-
- Criticality of the material to the overall performance / quality of the finished product.
-
-
-
- Functionality of the material in the drug product manufacturing and product efficacy.
-
-
-
- Regulatory commitments/ expectations.
-
-
-
- Supply history of vendor for a specific material.
-
-
-
- Quality history for a specific material.
-
-
-
- Audit history of the vendor.
-
-
-
- Based on above data report. Prepare the reduce testing proposal with change control for further approval.
-
Reduce Testing Plan
- Carry out the critical reduce testing parameters as per Annexure-1( but not limited to ).
-
-
-
- Change the parameter on the on the basis of risk assessment. (Wherever required)
-
-
-
- On receipt of the consignment, Check the containers physically for
-
-
-
-
- Proper labeling,
-
-
-
-
-
- Identification
-
-
-
-
-
- Intactness
-
-
-
-
- Verify the certificate of analysis received along with the consignment .
-
-
-
- If any of discrepancy found. Communicate to Head – QC or Designee.
-
-
-
- Head -QC shall communicate the discrepancies to the vendor and decide on the further course of action.
-
-
-
- Each container of the consignment shall be checked for a specific identification test preferably NIR /IR spectrum or any other suitable test as per the individual monograph.
-
-
-
- Section head QC officer or designee shall do the registration (Laboratory sample management software if available )for those tests which are mentioned in Annexure-1( but no limited to).
-
-
-
- The selected test parameters should have a mark of double asterisk ( * * ) in the respective raw material specification so as to provide information to the analyst during analysis.
-
-
-
Handling rejection of consignment(s) for Reduce Testing
- For raw material, if sample fails to meet the requirement of any test parameter, Investigate the failure as per the SOP on out of specification (OOS) investigation.
-
-
-
- On confirmation of the failure result, Analyze the consignment for the non-critical test parameter (i.e. other than reduce testing parameters) also.
-
-
-
- In case, the consignment fails to meet the requirement of any non-critical tests, Intimate to then Head – QC or Designee .
-
-
-
- Head – QC or Designee shall evaluate impact of the failure on previously released consignments and decide on further course of action.
-
-
-
- Take the subsequent consignments for complete analysis i.e. Do not apply the reduce testing, unless otherwise justified.
-
-
-
- On receipt of two consecutive rejected consignments, Transfer the vendor to “Under observation” and re-qualify as per the vendor qualification SOP.
-
-
-
Handling inconsistent supplies :
- In case consignments received from the vendor are of inconsistent quality e.g rejected consignment followed by approved consignment followed by rejected consignment, Head – QC or Designee shall intimate the vendor and decide on further course of action.
-
-
-
- In the course of action, Head – QC or Designee may decide on complete analysis of future consignments till three consecutive approved consignments received from the vendor.
-
-
-
- If three consecutive consignments are found satisfactory same as vendor’s COA, Consider the vendor as re-qualified and analyze further consignments for reduce testing.
-
-
-
Periodic verification for consistency of the quality :
- Every 10th Lot/ Batch, or Lot/ Batch received after 1 year from the release of first reduce tested consignment, whichever is earlier, Take for the complete analysis.
-
-
-
- Maintain a “Reduced testing tracking register” to track due for complete testing as per Attachment-1.
-
-
-
- Consider one consignment per year for complete analysis.
-
-
-
Reduce testing criteria for consignment received from other location of same company:
- On receipt of the consignment from the other location of own company, recipient laboratory shall sample the consignment as per the Raw material sampling SOP.
-
-
-
- If any test is not performed by vendor (other location), then sample the required quantity of the sample as per SOP for Sampling procedure of raw materials” for analysis of that particular test.
-
-
-
- Sample a minimum of 5 or √n + 1 No. of containers (whichever is higher), from the consignment of raw material and checked for a specific identification test separately.
-
-
-
- In case of material ( Pellets and granules) received from other location, Release the material on the basis of manufacturer’s COA.
-
-
-
- In exceptional case critical (e.g damaged containers received, wet containers received and tore label affixed on the containers). Perform the critical analysis .
-
-
-
- In case of material received, if manufacturer is same company other location and material is received after one year of manufacturing date then material shall analyze as per reduce testing criteria ( i.e. only Description and Identification).
-
8.0 ANNEXURE:
-
- Annexure-1: Reduce testing tracking register for raw material.
- Annexure-2 : Specific vendor master list for reduce testing.
- Annexure-3 : Critical reduce testing parameters register.
- Annexure-4 : Reduce testing proposal report.
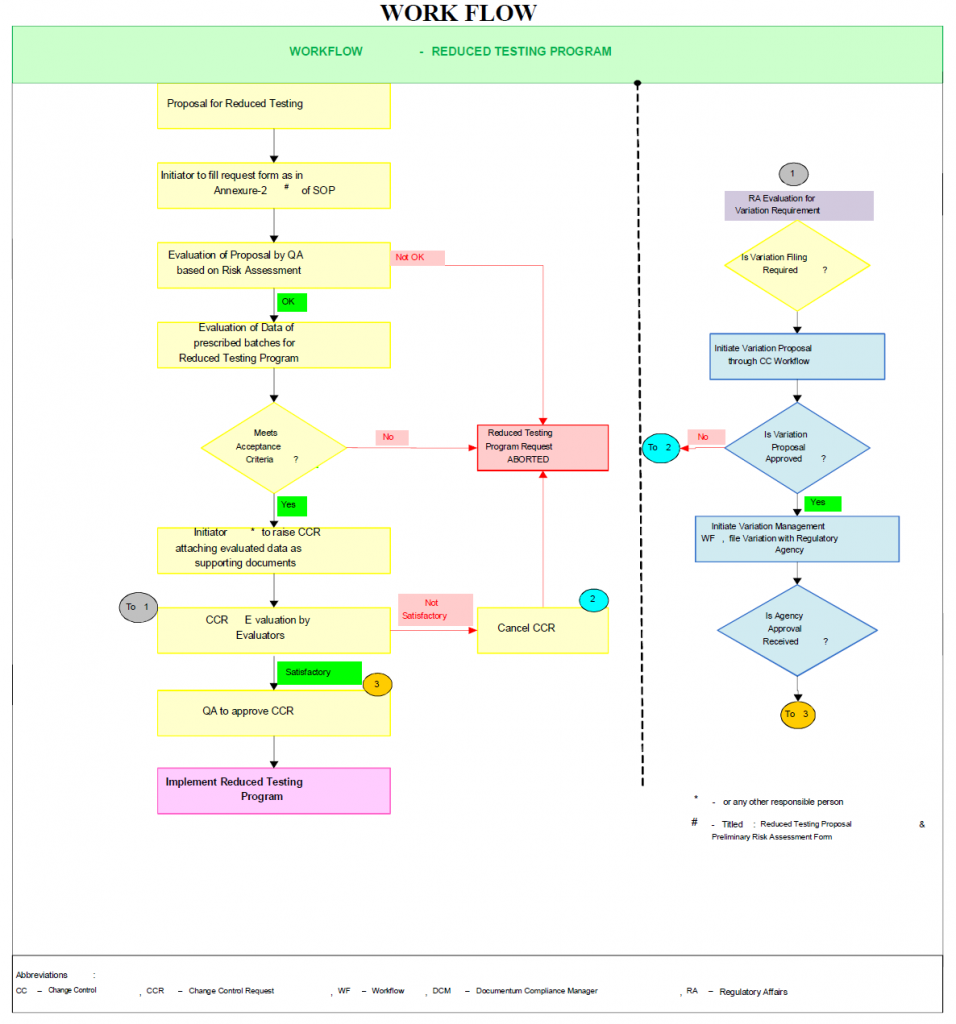
Flow Chart – Reduce Testing
REDUCE TESTING PROPOSAL AND PRELIMINARY EVALUATION FORM

PROTOCOL FOR EVALUATION OF BATCHES FOR REDUCED TESTING
<Insert name of API/Excipient/Packaging Material (as applicable)>
Material Code: ___________________________
Vendor Name: ___________________________
API/Excipient/Packaging Material Manufactured At: ___________________________
Specification Number: ___________________________
Document Number:________________________
Table of Contents
- Pre-Approval
- Background.
- Objective.
- Scope
- Procedure.
- Data Evaluation.
- Conclusions/Proposal for Reduced Testing.
- Post Approval
-
Pre-Approval:
-
- Signatures below indicate that this document has been prepared, reviewed and approved by persons functionally responsible for the tasks.
| Prepared by/ Function | Designation | Signature | Date |
| Reviewed By/ Function | Designation | Signature | Date |
| Approved By/ Function | Designation | Signature | Date |
-
Background:
-
- <Name of API/Excipient/Packaging Material> has been procured from <Name of Vendor> and is manufactured at <Mfg. location>.
-
- Analysis of the API/Excipient/Packaging Material(tick whichever is applicable) is done at the Vendor’s location before dispatch and a CoA is issued by the Vendor.
-
- Only approved material leaves the manufacturing premises of the Vendor. On receipt at <DF mfg location>, this API/Excipient/Packaging Material (tick whichever is applicable) is tested as per specifications before use in formulations.
-
Objective:
-
- The objective of this protocol is to evaluate data of batches of API/Excipient/Packaging Material (tick whichever is applicable) received at site.
- To identify test parameters that may be skipped on a batch-to-batch basis with the understanding that those batches not being tested still meet all established acceptance criteria.
- The objective of this protocol is to evaluate data of batches of API/Excipient/Packaging Material (tick whichever is applicable) received at site.
-
- Based on successful conformance, a Reduced Testing Program to be formulated whereby such tests to be performed only on pre-selected batches and/or pre-determined intervals.
-
Scope:
-
- This protocol can use for any API/Excipient/Packaging Material procured from approved Vendors and used at DF Manufacturing locations.
-
Procedure:
- Data of all the batches of the said API/Excipient/Packaging Material received at the site in a year shall be used for evaluation. However, the number of batches for this evaluation shall not be than 10 batches.
-
- For API/Excipients, batches used for the evaluation:
-
- Must be from an approved Vendor (and therefore, the Vendor must be one that fulfills all Vendor qualification criteria as detailed in SOP on Vendor Management)must be..
- From the same Vendor
- Must be from an approved Vendor (and therefore, the Vendor must be one that fulfills all Vendor qualification criteria as detailed in SOP on Vendor Management)must be..
-
-
- Consecutive lots received at from the vendor.
-
-
-
- Manufactured using the same process (Only for API)
-
-
-
- The same chemical composition ( Only for API)
-
-
-
Acceptance Criteria For API and Excipients:
- The said test parameters shall qualify for the Reduced Testing Program under the following conditions:
-
-
-
- Results of the test parameters intended to included in the Reduced Testing Program.
-
-
-
-
- For the batches evaluated comply with the specified limits as per current approved specifications at the organization as well as at the Manufacturer’s end (Should verify from the CoA provided by the Manufacturer).
-
-
-
-
- Methods used for testing at the Vendor’s end and the organization for the parameters to be included in the Reduced Testing Program are pharmacopoeial or validated.
-
-
-
- If the Vendor has excluded certain parameters from routine testing and has the relevant regulatory agency’s approvals for the same,
-
-
-
- The organization can include such parameters in its Reduced Testing Program, provided, all other conditions specified in this SOP are satisfied and an evaluation of the criticality of the reduced testing parameter with respect to the end use at the organization has been done.
-
-
- However, if the Vendor does not have necessary regulatory agency’s approvals for the Reduced Testing Program, the organization shall not include such parameters in its Reduced Testing Program.
-
- If the above said conditions are fulfilled, QA to provide approval for the requested parameters to be included in the Reduced Testing Program.
-
-
For Packaging Materials, batches used for the evaluation:
- (Followings are the mandatory requirement )
-
-
-
- An approved Vendor (and therefore, the Vendor must be one that fulfills all Vendor qualification criteria as detailed in SOP on Vendor Management)
-
-
-
- From the same Vendor
-
-
-
- Consecutive lots received at from the vendor
-
-
-
- Manufactured using the same process
-
-
-
- Must be of the same chemical composition.
-
-
-
Acceptance Criteria for Packaging Materials:
- The said test parameters shall qualify for the Reduced Testing Program under the following conditions:
-
-
-
- Results of all test parameters for the batches evaluated comply with the limits as per current approved specifications at the organization as well as at the Manufacturer’s end (which shall be verified from the CoA provided by the Manufacturer).
- None of the parameters which need to include in the Reduced Testing Program are out-of-trend or have significant change as per the relevant site SOPs for the same.
-
-
- Write Batch Nos. of the API/Excipient/ Packaging Material(tick whichever is applicable) used for evaluation:
-
Data Evaluation:
——————–
- Conclusions and Proposal for Reduced Testing:
7.1 Conclusion of Results
____________________________________________________________
7.2 Proposal for Reduced Testing
- Based on the above results, the following tests may include in the Reduced Testing Program:
| Sr.
No. |
Name of Test | Periodic Complete Testing to be done at (Frequency) |
7.3 Cautions to be exercised:
_______________________________________________________________
-
Post Approval:
-
- Signatures below indicate that this report has been prepared, reviewed and approved by persons functionally responsible for the tasks.



Pingback: SOP for Rounding off the Analytical Test Results - Pharma Beginners