The pharmaceutical industry is a vital sector that produces life-saving drugs and medical devices. However, the industry is not immune to human error, which can lead to costly consequences. Human error can occur at any stage of the pharmaceutical manufacturing process, from research and development to packaging and distribution. This article will discuss the causes and consequences of human error in the pharmaceutical industry and propose strategies to mitigate its impact.
Human Error Reduction : Best Practices and Strategies
- Human error is a significant challenge in the pharmaceutical industry, and its causes are multifaceted.
- Firstly, lack of training and education is a common cause of human error. Pharmaceutical manufacturing processes are complex and require specialized knowledge and skills. Without adequate training and education, employees may not understand the importance of following standard operating procedures or may not be aware of the potential risks associated with their actions.
- Secondly, the complexity of pharmaceutical manufacturing processes can also contribute to human error. The process involves multiple stages, from raw material sourcing to final product packaging, and requires strict adherence to quality control and assurance measures.
- Any deviation from the process can compromise the safety and efficacy of the final product. Lastly, fatigue and stress can also lead to human error in the pharmaceutical industry. Employees may work long hours or face tight deadlines, leading to exhaustion and reduced cognitive function. This can impair their ability to perform tasks accurately and safely.
- Human error in the pharmaceutical industry can have severe consequences, both financially and in terms of public health.
- Firstly, product recalls and market withdrawals are a common consequence of human error. These can result in significant financial losses for pharmaceutical companies and can also lead to shortages of critical drugs. Secondly, litigation and legal costs can also arise from human error in the pharmaceutical industry. If a product causes harm to a patient, the company may face lawsuits and regulatory penalties, leading to substantial legal costs and reputational damage.
- Lastly, human error can damage the brand reputation of pharmaceutical companies and erode consumer trust. The public relies on the pharmaceutical industry to produce safe and effective drugs, and any failure to meet this standard can lead to a loss of confidence in the industry as a whole.
Definition and types of human error in pharmaceutical
- In the pharmaceutical manufacturing industry, human error pertains to errors committed by personnel engaged in the manufacturing process of pharmaceuticals. Serious repercussions from these mistakes may include product recalls, injuries, and even fatalities. Comprehending the many categories of human error helps facilitate the identification of possible problems and enhance safety protocols in the production process.
-
Human error falls into a number of categories in the production of pharmaceuticals, including:
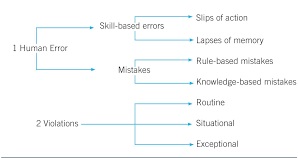
- Slips and Lapses: These happen when someone inadvertently veers off the desired path of action. While lapses are typified by a transient memory loss, slips include a brief interruption in the performance of a task. Errors with medication, inaccurate labeling, and equipment faults are a few examples of slips and lapses.
- Mistakes: Errors are the result of someone purposefully carrying out a task but making a mistake. These mistakes can be divided into additional groups:
- Memory errors: These are blunders brought on by a person’s inaccurate memory of facts. Examples include miscalculating dosages or reading directions incorrectly.
- Rules errors: In this kind of error, a person interprets a rule or procedure incorrectly or applies it incorrectly. Incorrect ingredient mixing or production step sequencing are two examples.
- Errors in strategy: These mistakes happen when someone chooses an inadequate or inefficient approach for a task. Examples include selecting the incorrect machinery for a given procedure or neglecting to account for any problems that can occur during manufacturing.
- Violations: When someone purposefully departs from set protocols or rules, usually because they believe they are short on time or resources, this is called a violation. Violators may omit necessary quality control inspections or use materials that are not authorized during manufacture.
- Organizational influences: Inadequate training, shoddy communication, and a lack of resources are just a few examples of organizational elements that might have an impact on human error in the pharmaceutical manufacturing process. These elements can raise stress levels, lower motivation, and lower general job satisfaction, all of which can lead to mistakes.
The consequences of human error on drug quality, safety, and efficacy
- In order to reduce human error in pharmaceutical manufacturing, it is critical to have strong quality control procedures in place, give staff members extensive training and assistance, and promote an environment that values accountability and ongoing development.
-
Introduction
- Human error can have significant consequences on the quality, safety, and efficacy of drugs. The pharmaceutical industry is highly regulated to ensure that drugs are safe and effective for use by patients. However, mistakes made during various stages of drug development, manufacturing, and administration can compromise the quality and safety of medications. This article will explore the consequences of human error on drug quality, safety, and efficacy in detail.
-
Drug Development
- Drug development is a complex process that involves multiple stages, including discovery, pre-clinical testing, clinical trials, regulatory approval, and post-marketing surveillance. Human error at any stage can have far-reaching consequences.
- During the discovery phase, scientists identify potential drug candidates based on their therapeutic potential. However, errors in data analysis or interpretation can lead to the selection of inappropriate candidates or the dismissal of promising compounds. This can result in wasted resources and delay the development of effective drugs.
- In pre-clinical testing, drugs are evaluated for safety and efficacy using animal models. Errors in experimental design or data collection can lead to inaccurate results, potentially leading to false conclusions about a drug’s safety profile. Such errors may result in the advancement of unsafe drugs into clinical trials or the discontinuation of potentially beneficial compounds.
- Clinical trials are critical for assessing a drug’s safety and efficacy in humans. Human error during trial design or execution can compromise the reliability of study results. Errors may include inadequate sample sizes, biased participant selection, improper randomization, or flawed data collection methods. These errors can lead to incorrect conclusions about a drug’s effectiveness or safety profile.
-
Manufacturing
- Once a drug receives regulatory approval, it enters the manufacturing phase. Human error during manufacturing can have severe consequences on drug quality and safety.
- Errors in formulation or compounding can result in inconsistent drug potency or inconsistent release profiles. Inadequate mixing of ingredients may lead to non-uniform distribution of active pharmaceutical ingredients (APIs) within a dosage form, affecting drug efficacy. Contamination during manufacturing can introduce impurities or foreign substances, compromising drug safety.
- Inaccurate labeling or packaging can result in medication errors. If drugs are mislabeled, incorrect dosages may be administered to patients, leading to potential harm. Similarly, errors in packaging can result in the wrong drug being dispensed to patients, further increasing the risk of medication errors.
-
Prescribing and Administration
- Human error can also occur during the prescribing and administration of drugs, further impacting drug safety and efficacy.
- Prescribing errors may involve incorrect dosage calculations, inappropriate drug selection, or misinterpretation of medication orders. These errors can lead to patients receiving suboptimal doses or being exposed to drugs that are contraindicated for their condition. Such errors may compromise treatment outcomes and patient safety.
- Medication administration errors can occur in various healthcare settings, including hospitals, clinics, and home care. Errors may involve administering the wrong drug, incorrect dosage, or improper route of administration. These mistakes can result from miscommunication, distractions, or inadequate training. Medication administration errors can have serious consequences on patient health and may lead to adverse drug reactions or treatment failure.
-
Consequences
- The consequences of human error on drug quality, safety, and efficacy are significant and multifaceted.
- Patient Harm: Errors in drug development, manufacturing, prescribing, or administration can directly harm patients. Substandard drugs may fail to treat the intended condition effectively or cause adverse reactions. Medication errors can result in incorrect dosing or administration routes, leading to treatment failure or harm to patients.
- Regulatory Consequences: Human errors that compromise drug quality or safety can have legal and regulatory implications. Regulatory agencies such as the Food and Drug Administration (FDA) impose strict guidelines on pharmaceutical companies to ensure drug quality and safety. Failure to meet these requirements due to human error can lead to regulatory actions such as recalls, fines, or even the suspension of manufacturing operations.
- Financial Impact: Human errors in drug development, manufacturing, or administration can have significant financial implications. The cost of wasted resources during drug development, such as failed clinical trials or discontinued compounds, can be substantial. Medication errors may result in additional healthcare costs due to adverse reactions or the need for corrective treatments.
- Loss of Public Trust: Human errors that compromise drug quality and safety erode public trust in the pharmaceutical industry. Incidents of substandard drugs or medication errors can create skepticism among patients and healthcare providers. Restoring public confidence can be challenging and may require increased transparency, improved quality control measures, and enhanced regulatory oversight.
-
Conclusion
- Human error at various stages of drug development, manufacturing, prescribing, and administration can have far-reaching consequences on drug quality, safety, and efficacy. Errors during drug development can delay the availability of effective medications or lead to the advancement of unsafe drugs. Mistakes during manufacturing can compromise drug quality and introduce safety risks. Prescribing and administration errors can directly harm patients and undermine treatment outcomes. Understanding the potential consequences of human error is crucial for implementing robust quality control measures, improving training programs, and enhancing regulatory oversight to ensure safe and effective medications reach patients.
Best Practices and Strategies for Minimizing Human Error in Pharmaceutical
- Human error is a significant concern in the pharmaceutical industry, as it can lead to inaccurate drug formulations, contamination, and ultimately, patient harm. To minimize human error, various best practices and strategies should be implemented across all stages of drug production and distribution. Here are some key approaches:
-
In today’s fast-paced and highly competitive industries, ensuring quality control is crucial for businesses. Quality control laboratories play an essential role in this process, as they are responsible for monitoring and verifying the integrity and reliability of products. However, human error in these laboratories can lead to significant consequences, including inaccuracies in test results and compromised product safety. Therefore, it is imperative to develop effective strategies to minimize human error and enhance the reliability of quality control laboratories.
-
Standard Operating Procedures (SOPs):
- Implementing well-designed Standard Operating Procedures is fundamental in minimizing human error. These SOPs provide step-by-step guidelines for laboratory operations, ensuring consistency in testing procedures and reducing the likelihood of mistakes. Laboratories should regularly review and update SOPs to reflect any advancements or changes in the industry.
-
Training and Education:
Investing in comprehensive training and education is crucial to minimizing human error. Laboratory personnel should receive proper training on relevant techniques, instruments, and protocols to enhance their skills and knowledge. Regular workshops and seminars should be arranged to keep employees updated with the latest developments and best practices in the field.
-
Error Reporting and Investigation:
Establishing a reliable error reporting system encourages laboratory professionals to report any errors or near-misses. These reports should be followed by a thorough investigation to identify the root causes and implement corrective actions. Promoting a blame-free environment encourages staff to report errors without fear of retribution, allowing for a collective learning experience and continuous improvement.
-
Automation and Technology:
Leveraging automation and advanced technology can significantly minimize human error in quality control laboratories. Employing automated instruments and robotics reduces manual intervention and the associated risks. Additionally, utilizing specialized software and data management systems can improve accuracy, traceability, and efficiency in laboratory processes.
-
Quality Control Checks:
Implementing stringent quality checks within the laboratory is critical in reducing human errors. These checks, such as parallel testing, random sample retesting, and blind controls, help identify discrepancies and ensure the accuracy and precision of test results. Regular calibration and maintenance of laboratory equipment also contribute to minimizing errors and maintaining measurement accuracy.
-
Workload Management:
Managing the workload of laboratory personnel is vital to preventing human error. Overburdening employees with excessive workloads can result in fatigue, stress, and reduced attention to detail. Dividing responsibilities appropriately and employing a team approach to complex tasks can distribute the workload evenly and reduce the risk of errors.
-
Continuous Validation and Verification:
Regular validation and verification of laboratory methodologies and processes are essential to maintaining accuracy and minimizing human error. Conducting inter-laboratory comparisons, proficiency testing, and internal audits helps identify and rectify any discrepancies. Continual monitoring and improvement can enhance the reliability of laboratory operations.
-
Document Control and Review:
Maintaining a robust document control system ensures that laboratory protocols, procedures, and guidelines are up-to-date and accessible. Regular reviews and revisions of documents enable laboratories to adapt to evolving industry standards and best practices. Additionally, incorporating electronic data management systems facilitates efficient documentation and reduces the risk of errors associated with manual record-keeping.
-
Continuous Professional Development:
Promoting continuous professional development is fundamental in enhancing the competency and expertise of laboratory personnel. Encouraging participation in workshops, conferences, and relevant courses enables employees to remain updated with emerging technologies and advances in quality control. By aligning their skills with the latest industry trends, professionals can minimize potential errors.
-
Effective Communication and Collaboration:
Encouraging a culture of effective communication and collaboration between laboratory personnel is essential to minimizing errors. Regular team meetings, open discussions, and interdisciplinary collaborations foster knowledge sharing, problem-solving, and error prevention. By enabling effective communication channels, laboratories can identify and address potential errors before they become significant issues.
-
Conclusion:
Minimizing human error in quality control laboratories is crucial to ensuring reliable products and maintaining the trust of the consumers. By implementing strategies such as SOPs, training and education, error reporting systems, automation, quality control checks, workload management, continuous validation, document control, professional development, and effective communication, laboratories can significantly reduce human errors. This concerted effort toward error prevention will ultimately enhance the accuracy, efficiency, and overall quality of laboratory operations.


