Standard Operating Procedure for Dispensing of Raw Material (API-Active Pharmaceutical Ingredient and Excipient) to the production department for the manufacturing of pharmaceutical drug products.
1.0 PURPOSE:
-
- The purpose of this SOP is to define the procedure for dispensing of Raw Materials.
2.0 SCOPE:
-
- This procedure is applicable for dispensing of Raw Materials, in the pharmaceutical manufacturing plant.
3.0 REFERENCES:
-
- In House

- In House
-
- Partly Dispensed Material Control SOP
-
- Cleaning and operation Laminar Air Flow- SOP
-
- Receipt, Storage & dispensing of solvents – SOP
-
- The procedure of Type A and Type B Cleaning – SOP
-
- Clearance of lines, area, and equipment- SOP
-
- Environmental Monitoring – SOP
4.0 RESPONSIBILITY – (SOP for Raw Material Dispensing):
-
- Officer of the warehouse shall be responsible for following the procedure of dispensing of raw material and take the line clearance of the area and equipment, with QA Personnel.
-
- Head of the department/designee of warehouse & QA Head shall be responsible for reviewing and implementation the SOPs.
-
- Production Personnel shall provide the work order issue (Pick up List) to the warehouse department.
-
- QA shall be responsible for checking the Material Requisition Order (MRO) provided by the production.
-
- Verify the raw materials after staging.
-
- To provide the line clearance of area and equipment before raw material dispensing.
-
- To ensure the warehouse person carry out the raw material dispensing as per SOP.
5.0 ABBREVIATIONS:
-
- BMR: Batch Manufacturing Record
-
- MIO: Material Issue Order
-
- MRO: Material Requisition Order
-
- QA: Quality Assurance
-
- RLAF: Reverse Laminar Air Flow
-
- RMS: Raw Material Store
-
- SOP: Standard Operating Procedure
-
- SS: Stainless Steel.
6.0 PROCEDURE:
-
-
MIO/MRO Generation and verification :
- Production Officer shall generate the Material Issue Order (Pick Up List) through Metis System/Other Software/Manual.
-
-
- Generate the material issue order (MIO) in such a manner to follow the FEFO (First Expiry First Out) basis or FIFO (First in First Out) in case non-availability of the expiry date.
-
- In case of any trial batches, through consumption issue, deviated issue and on-demand issue, if required, any particular Analytical Report No (AR No.) of any material shall be picked up manually in Metis System/Other Software/Manual.
-
- MIO shall be check (i.e. Material Name, Quantity, Item Code and A.R. No., etc. by production & warehouse personnel and verified by IPQA officer with respect to BMR.
-
- If discrepancy found, at any stage of verification it shall be returned to concern production personnel for its rectification.
-
- Production personnel shall rectify the MIO with the consultation of IT department.
-
- After rectification, MIO shall be handed over to IPQA officer for verification.
-
- IPQA officer shall verify the MIO with respect to BMR. If found satisfactory then it shall be handed over to production personnel for further processing.
-
- Production personnel shall hand over the MIO to Warehouse (RMS) department for staging and dispensing of raw material.
-
-
Materials Staging and Verification :
- Store officer shall generate the staging materials description details paper from Metis System/ERP/Other Software/Manual, and allow the workmen to collect the materials from different locations according to staging paper and keep the materials on pallets except for purified water in the staging area.
-
-
- Note: Purified water shall be dispensed by production personnel in the production area.
-
- After completion of staging of materials, all staged material shall be checked by the warehouse officer and cross-verified by QA officer with MIO.
-
- If found satisfactory, warehouse personnel shall generate the material issue coupons from the Metis System/ERP/Other Software/Manual.
-
-
Cleaning and Checkpoints :
- Warehouse personnel shall clean the area and equipment as per the SOP No. BSPN/003
-
-
- The warehouse officer shall start the RLAF at least 20 minutes before the start of the raw material dispensing activity.
-
- After completion of the cleaning, activity check the following checkpoints.
-
- Cleanliness of Dispensing Booth, RLAF and Balances.
-
- Performance check of balance with the calibrated standard weight, Record of the Calibration.
-
- Check the Humidity & Temperature of dispensing booth and maintain the record.
-
- Check the Differential Pressure of Reverse Laminar Airflow & maintain the record.
-
- Availability of the required number of cleaned utensils.
-
- Check the availability of the required number of cleaned containers for store the dispensed materials.
-
- Check the availability of the required number of cleaned secondary over-gowns.
-
-
Line Clearance Procedure :
- The warehouse officer shall fill the checklist as per SOP Clearance of lines, area, and equipment for taking the line clearance from QA personnel.
-
-
- QA shall check the Dispensing booth and give the line clearance for starting the dispensing activity.
-
- Warehouse officer shall take a printout of the status label containing Product Name, Batch Number, Lot No., Container No. (Container No. write manually on the status label after dispensing), etc.
-
- Status label affix on the container which shall be used for dispensed material.
-
-
Dispensing of Raw materials:
- Store officers and workmen shall wear secondary gown before starting the dispensing activity in the dispensing booth.
-
-
- Warehouse officer shall segregate the material issue coupon according to their dispensing sequence and dispense the materials (Active Ingredients and excipients) as per given sequence- first dispense excipients, followed by fluffy materials like (Colloidal silicon dioxide/Sodium lauryl sulfate, etc.), then dispense color, then clean the dispensing booth as per “TYPE-B” cleaning and then dispense Active Ingredients.
-
- After completion of line clearance activity transfer the staging material in the dispensing booth one by one as per the sequence.
-
- During dispensing, if any materials are not pickup by the Metis System/ERP/Other Software/Manual in the above-said sequence then dispense the materials as per the below sequence.
-
- First, dispense Active Ingredients then excipients, followed by fluffy materials like (Colloidal Silicon Dioxide/Sodium Lauryl Sulphate, etc.,), then dispense color.
-
-
Dispense solvent in solvent dispensing area only (Refer SOP for Solvent Dispensing)
- Assemble the container/bags that are to be used for dispensing in a suitable sequential order.
-
-
- Use cleaned and dried raw material dispensing equipment (Scoops, Spoons, Spatula, SS Tanks, etc.) and fresh hand gloves for each raw material for dispensing.
-
- Use two transparent poly bags for dispensing of each raw material. In case of light-sensitive materials, use three polybags i.e. inner transparent, middle black and outer transparent polybag.
-
- Note: In case of light-sensitive materials tare of polybag shall be done of only two polybags (inner transparent & middle black).
-
- Dispense one material at one time for a particular product “In case any product having the same strength, batch size, a maximum of five batches can be dispensed at one time”.
-
- After dispensing of five batches, Type B cleaning of the dispensing room should be done as per SOP for Type A & Type B Cleaning.
-
- Ensure light-sensitive material to be dispensed in monochromatic light as per Annexure-3 “List of Light-Sensitive Material”.
-
- In case of material required Nitrogen flushing, mentioned as per Annexure-4 “List of Nitrogen Flushing Material”, purge the Nitrogen in the dispensed container and close the inner bag.
-
- The parent container from which material dispensed shall also be purge with Nitrogen.
-
- Scan all the materials with Barcode Scanner of each raw materials mention in Work order issue (Pick up the list) before raw material dispensing.
-
-
Weighing of raw materials:
-
-
- Tare the polybags by putting polybags on the weighing balance platform followed by pressing the Tare Key.
-
- Check the display of the weighing balance that is showing zero after tarring.
-
- Record the tare weight in Material Issue Coupon.
-
- Weigh the required quantity (Net Weight) of material against MRO.
-
- Check net quantity in the display of weighing balance against the quantity in MRO.
-
- Adjust the required quantity of weighing material by adding/removing in it during its weighing activity.
-
- Remove the material from the balance platform and press the Tare Key to set zero and check zero in balance display.
-
Once again weigh the materials to get the gross weight (Tare weight & Net weight)
- Add the tare weight with net weight in Material Issue Coupon to get the gross weight.
-
- Verify the displayed gross weight with Material Issue Coupon gross weight and put the sign on coupon.
-
- Ensure that the displayed value of gross weight of balance is within the tolerance limit of that balance.
-
- Place the material issue coupon in between the two polybags which can be seeing externally.
-
- Tie the dispensed materials, by twisting the open mouth of polybag in case of solid materials and tightly closed the cap/lid in case of liquid materials.
-
- Keep dispensed material in the clean containers having the status of operation and batch No.
-
- Transfer the dispensed materials to post raw material dispensing staging area by putting it on clean pallets.
-
- Close the raw material container properly and affix “Partly dispensed label” as per SOP for “Partly dispensed material control”, remove the container from the dispensing room and arrange it on the pallet outside the dispensing room.
-
-
Dispensing of Intact Container – Raw Material
- For dispensing of the raw material intact container on the receipt of material pick up a list from the production department follow the above procedure.
-
-
- Clean intact containers with a dry clean cloth before taking it in the dispensing area/post staging area.
-
- Write down the Gross weight, Net weight and Tare weight on material issue coupon. Gross weight shall be taken by weighing of the intact container, net weight. from the mother label of container and Tare weight by subtraction of net weight from the gross weight.
-
- Note: Weighing balance ID shall also be written in the material issue coupons.
-
Place the material issue coupon in between the two polybags which can be seen externally.
- Dispensing of empty hard gelatin capsules by taking Manufacturer/Supplier’s Net weight mentioned in Kgs. on the outer label of each box to be multiplied with quantity to be dispensed divided by quantity mentioned in numbers by Manufacturer/Supplier’s on outer label of each box.
-
- After scanning & weighing of all materials all materials confirm the same work order issue in Metis System/ERP/Other Software/Manual and take the print out of confirm copy of the work order issue (Pick up list) and keep it in respective BMR.
-
- Print the “Dispensed Material Label” as per annexure-2 and affix it on each dispensed materials container/ SS tank.
-
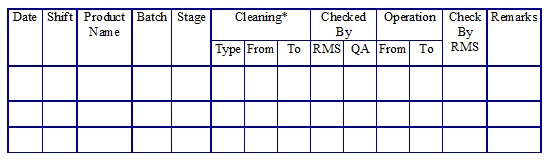
- Maintain the records in RM Dispensing Area sequential Log.
-
- Rearrange the leftover raw materials to their respective place as per location code mentioned on partly label after completion of the dispensing activity.
-
- Production officer & IPQA officer shall counter check the materials before starting the manufacturing operation in the production area.
-
- Note: Intact Container could be dispensed in the post staging area.
7.0 ANNEXURES:
RM Dispensing area sequential Log. (Annexure 1)
Dispensed Material Label (Annexure 2)
Product :
Batch No : Operation :
Stage : Lot :
Container No :
Material Dispensed By :
Material Dispensed Date : Work Order Issue No:
Light-Sensitive Material List. (Annexure 3)
| Sr. No. | Item Code | Name of the Material |
List of Nitrogen Flushing Material. (Annexure 4)
| Sr. No. | Item Code | Name of the Material |
List of Raw materials for dispensing in intact containers. (Annexure 5)
| Sr. No. | Item Code | Name of the Material |



Pingback: Dispensing Area : Cleaning & Riser Filters Replacement -