Performance Qualification Protocol for sterilizing and depyrogenating tunnel Dry Powder Injection Production area.
Qualification (PQ) of Sterilizing and Depyrogenating Tunnel
1.0 Objective:
- The purpose of this protocol is to provide an outline for the qualification of the Sterilizing and Depyrogenating Tunnel by verification of performance attributes such as:
-
- The PAO Penetration through installed HEPA Filters, joints, and flanges in Sterilizing and Depyrogenating Tunnel meets the limits as described in the acceptance criteria.
-
- Air Velocities at the surface/face of HEPA Filters in Sterilizing and Depyrogenating Tunnel meet the limits as laid down in acceptance criteria.
-
- Pressure Differentials of all the zones meet the limits as laid down in acceptance criteria.
-
- The Air Flow Pattern in the Sterilizing and Depyrogenating tunnel meets the requirements laid down in acceptance criteria.
-
- The Sterilizing and Depyrogenating Tunnel meets the Class 100 requirements for non-viable and viable particle count as per acceptance criteria.
-
- The temperature distribution in Sterilizing and Depyrogenating tunnels during empty chamber runs should be as per the acceptance criteria.
-
- The heat penetration within the intended loads should meet the requirements of the acceptance criteria.
-
- The process lethality and endotoxins reduction provided by the Depyrogenating cycle meet the requirements of the acceptance criteria.
2.0 Scope:
-
- This protocol covers all aspects of Performance Qualification for the Sterilizing and Depyrogenating tunnel I.D. No. _______ installed in vial washing and sterilization of dry powder injection line – 03 facility.
-
- Reference Document:
Following documents are referred to during the preparation of the protocol.
| Document Name | Document Number |
| Operational Manual | |
| Operation Qualification Report | |
| SOP of operation & Cleaning of Depyrogenating Tunnel | |
| Operation procedure of Air sampler (Viable Particle) | |
| SOP on environmental monitoring of the sterile facility | |
| SOP on bacterial Endotoxin (LAL) Test |
-
-
Equipment Description:
- Use: The Sterilizing and Depyrogenating tunnel is used to dehydrogenate and sterilize the glass vials under high thermal conditions.
-
-
- The capacity of Equipment: Conveyor width XY mm
-
- Name of the Equipment:

- Name of the Equipment:
-
- Make of the Equipment:
-
- Model No.:
-
- Serial No.:
-
- Equipment ID No.:
-
- Location of the Equipment:
-
- Basic Design Features of Equipment:
-
- Basic operation features:
3.0 Responsibility – Qualification of Sterilizing and Depyrogenating Tunnel :
-
- Responsibilities of different department/ personnel involved in different activities related to the performance qualification of Sterilizing and Depyrogenating Tunnel are defined below:
-
- Quality Assurance (Validation):
-
- Preparation and review of PQ protocol.
-
- Approval of PQ protocol.
-
- Execution of PQ protocol along with the co-ordination of other departments.
-
- Review of results and compiling of reports.
-
- Preparation and approval of PQ report.
-
- Production:
-
- Checking of PQ protocol and Report.
-
- Execution of PQ protocol along with QA validation team.
-
- Quality Control:
-
- Performing testing
-
- Preparation of Analysis Report and submission to Quality Assurance
-
- Engineering:
-
- Preventive Maintenance of Sterilizing and Depyrogenating Tunnel as per schedule
-
- Rectification of Breakdown during qualification study.
4.0 Qualification Test Method:
-
- Pre-Requisites:
-
- Prior to conducting/ executing the Performance qualification protocol following conditions must be fulfilled:
-
- Equipment and System should be safe for execution.
-
- Verification of standard operating procedure and other relevant documents.
-
- Maintenance of required environment specifications in operational areas.
-
- Completion of training and associated documentation of all personnel involved in the qualification exercise.
-
- All loading items must be ready for dehydrogenation as per relevant SOP.
-
- The pre-checks and reconciliation of endotoxin vials are to be verified in the test datasheet enclosed as Form 05 & 06 as per the table given below.
| Sr. No. | Pre checks |
| 1. | Check for any fault in the current cycle that is taken in the Sterilizing and Depyrogenating Tunnel. Verify the PLC printouts and strip charts data. |
| 2. | Check the calibration status of the measuring instrument fitted to the Tunnel. |
| 3. | Check the loads prepared and loading pattern as per protocol. |
| 4. | Check for any major maintenance activity carried out on the tunnel recently. |
| 5. | Check for the tunnel program that is to be validated. Verify the parameter printout. |
| 6. | Check the endotoxin vial. |
| 7. | Check the thermocouples for sensitivity by dipping in hot water and cold water and observing the temperature change. |
| 8. | Check the endotoxin spiked vials before loading them onto the tunnel. |
-
Pre-Qualification test for Sterilizing and Depyrogenating Tunnel:
-
- Calibration of all measuring, controlling, and recording instruments of Sterilizing and Depyrogenating Tunnel.
-
- Calibration of Test instruments.
-
- Verification of Conveyor speed.
-
Qualification Test Program:
-
- Integrity Testing of HEPA Filters (By External Agency).
-
- Air Velocity Measurement Studies.
-
- Air Flow Pattern Studies.
-
- Differential Pressure Monitoring.
-
- Non-Viable Particle monitoring.
-
- Viable particle monitoring (settle plate & active air sampling)
-
- Empty Tunnel Heat Distribution Studies.
-
- Loaded Tunnel Heat Penetration Studies.
-
- Endotoxin Challenge Studies.
-
- Calculation of Value.
-
Test Matrix:
The following matrix shall be followed for the initial qualification and re-qualification of Sterilizing and dehydrogenating tunnel for its performance:
| Sr. No. | Test | Frequency | Other Requirement | |||
| Initial Qualification | Re –
Qualification |
|||||
| Pre – Qualification Test | ||||||
| 1 | Calibration of Instruments of Depyrogenating Tunnel | Once | Once | Before Qualification | ||
| 2 | Calibration of Test Instruments | Once | Once | Calibration certificate of secondary standards | ||
| 3 | Verification of Conveyor Speed | Thrice | Once | — | ||
Qualification Test |
||||||
| 1 | Integrity Testing of HEPA Filters | Thrice | Once | Before Qualification | ||
| 2 | Air Velocity Measurement Studies | Thrice | Once | —– | ||
| 3 | Air Flow Pattern Study | Once | Once in Two Year | As per schedule/procedure | ||
| 4 | Differential Pressure Monitoring | Once | Once | — | ||
| 5 | Non Viable Particle monitoring | Thrice | Once | ——— | ||
| 6 | Viable particle monitoring | Thrice | Once | For three days during the initial qualification | ||
| 7 | Empty Tunnel Heat Distribution Studies | 3 runs | One run | NA | ||
| 8 | Loaded Tunnel Heat Penetration Studies | 3 runs | One run | Requalification as per annexure No. 12 | ||
| 9 | Endotoxin Challenge Studies | All thermometric studies of loaded tunnel heat penetration studies of different vial sizes. | ||||
| 10 | Calculation of FH Value | All thermometric studies of the empty tunnel and loaded tunnel heat penetration studies of different vial sizes. | ||||
| 11 | Physical defects checks | In all heat penetration study runs. | ||||
| 12 | Cooling chamber sterilization cycle | 3 runs | One run | NA | ||
Note: The test matrix or test replications may be modified for any change or need basing on suitable Risk assessment. A suitable justification and risk assessment report should be attached with the Performance Qualification Report.
Scientific rationale for selection of vial type:
-
- The molded vials have a higher weight and more surface thickness as compared to the tubular vials of the same size and hence will have higher resistance to heating and slow heat transfer. This is the reason for which the molded vials are taken as the load for the performance qualification.
-
- The molded vials have greater body surface area as compared to the tubular vials of the same size and hence surface covering probability in molded vials is less. This is the reason for which the molded vials are taken as the load for the performance qualification.
-
- Considering the molded vials as worst case it is taken as the load for the performance qualification.
-
Qualification Procedure of Sterilizing and Depyrogenating tunnel:
-
- Prequalification test procedure:
-
-
- Calibration of Instruments of Sterilizing and Depyrogenating Tunnel:
-
All measuring, controlling, and recording instruments of the sterilizing and dehydrogenating tunnel must be calibrated.
| Sr. No. | Item Description |
| 1 | Loop calibration of all sensors attached to the Inlet, Sterilization, and Cooling chambers. |
| 2 | All Magnehelic and Photophilic Pressure Gauges attached to the Sterilizing and Depyrogenating Tunnel. |
| 3 | Loop calibration of Temperature Transmitters. |
Observations and Results:
Verify and attach all calibration certificates with the report.
| Sr. No. | Item Description | Calibration Plan |
| 1 | Data logger | Verify calibration before qualification |
| 2 | All the additional sensors are used for performance qualification. | Calibrate before the qualification study and calibration should be verified at the end of the qualification study. |
| 3 | Particle counter | Verify calibration before the test |
| 4 | Anemometer | Verify calibration before the test |
| 5 | Stop Watch | Verify calibration before the test |
| 6 | Active Air Sampler | Verify calibration before the test |
| 7 | Aerosol Photometer | Verify calibration certificate and traceability (external agent) before the test. |
| 8 | Liquid Particle counter | Verify calibration before the test |
Acceptance Criteria:
All thermocouples used for Qualification should be Calibrated within + 0.5 0C of the high reference temperature before and after execution. 95 % of the Thermocouples used must be in operation during post verification. All critical thermocouples (used in a cold spot and adjacent to controlling sensor must be operational after Qualification study.
Note: The 95 % criteria is used to allow flexibility of operation so that if some sensors cease to function as intended during the run the whole study is not affected. But these sensors should not be the critical ones as that used for measuring the temperature near the controlling probe or that in the cold spot or any other critical locations. In that case, the study needs to be repeated after proper investigation and corrective action.
Observations and Results:_____________________________________________________
Verify and attach all calibration certificates with the report.
-
Verification of Conveyor Speed:
Objective:
The objective of this test is to demonstrate that the conveyer speed of Sterilizing and Depyrogenating Tunnel meets the acceptance criteria.
Test Requirements: Calibrated Stopwatch.
Measuring Scale/Tape.
Test Procedure
Measure 200 mm length of a conveyer belt with the help of measuring tape and record in Test datasheet enclosed as Form No. 01.
Mark the conveyor belt with a permanent marker such that half of the mark is on the conveyor belt and the rest half on the support guard of the conveyor belt. Set the conveyor speed on the HMI and start the conveyor. Simultaneously start the stopwatch. Measure the time (T) in second for traversing 200 mm distance as marked on the conveyer belt.
Calculate the Velocity (V) of the Conveyer as follows
V (mm/Min.) = {200(mm) x 60 Sec.} / T Sec.
Perform the test with all the speeds as defined in the Test datasheet enclosed as Form No. 01.
Note:* As per manufacturer recommendation with reference to operational manual (Ref. No. XYZ).
Acceptance Criteria:
The Conveyer speed in HMI of Sterilizing and Depyrogenating Tunnel and the reading observed should not vary ± 2%.
Results:
Record the observations in the Test datasheet enclosed as Form No. 01.
Qualification Test Procedure:
Integrity Testing of HEPA Filters (By External Agency):
Objective:
To establish the integrity of the HEPA Filters, Joints, and flanges installed in the Sterilizing and Depyrogenating Tunnel.
Test Requirements:
- Calibrated Aerosol Photometer and scanner.
- Compressed air/Nitrogen source.
- Aerosol with quality certificate.
- Technical Agreement with the External agency / Certificate of competence of the testing personnel of the external agency.
-
Test Procedure:
- The temperature of Sterilizing Zone of the Tunnel should not be more than 5°C above the ambient temperature at the start of the test.
- Switch “ON” the Blowers of the Sterilizing and Depyrogenating Tunnel. Switch “OFF” the heaters. Let the blowers run for 15 minutes for Zone’s stabilization.
- Directly insert the test aerosol from the aerosol port provided in the tunnel of all 3 Zones of Sterilizing and Depyrogenating Tunnel by using nitrogen or compressed air at test pressure not less than 1.5 kg/cm2.
- Switch the Aerosol Photometer “ON” and allow stabilizing for five minutes.
- Adjust the aerosol generation to achieve an Aerosol challenge concentration of approximately 20-80 µg/liter of air. Adjust this challenge concentration upstream of the HEPA filter (100% aerosol port) and check for detection in the photometer.
- Scan the HEPA Filter surfaces and periphery including fittings, flanges, and joints bypassing the receptor probe 1 inch from the filter surface, in overlapping strokes for any leakage.
- If there is any leakage from fittings, flanges, and joints then take appropriate corrective action and repeat the test. The leakage quantity and the corrective action must be recorded.
- If there is damage to filter media then sealant can be applied to repair the damage but that should not be more than 3% of the filter face area (not including the frame). Ref: SOP. The test should be repeated after repair.
- Acceptance Criteria:
- The Aerosol penetration/leak through HEPA filters should be less than 0.01% of the upstream Aerosol concentration.
- Results:
- Verify the results and attach the report.
-
Air Velocity Measurement Studies:
Objective:
To demonstrate that the air system is balanced and capable of delivering sufficient air volumes to maintain a minimum cross-sectional velocity under the HEPA filters measured 6 inches downstream of the HEPA filters of all the zones as per the acceptance limit mentioned below.
Test Requirements: Calibrated Anemometer.
Test Procedure:
- The test shall be carried out as per Test Matrix.
- The temperature of Sterilizing Zone of the Tunnel should not be more than 5°C above the ambient temperature at the start of the test.
- Switch “ON” the Blowers of the Sterilizing and Depyrogenating Tunnel. Keep the Heaters “OFF”. Let the blowers run for 15 minutes for Zone’s stabilization.
- Measure the velocity in all HEPA Filters installed in all the zones of the Depyrogenating Tunnel with an Anemometer probe, 6 inches from the filter face.
- Note down the velocities at the locations shown in Figure-1 of each HEPA filter. Measurements should be taken for a minimum of 15 seconds.
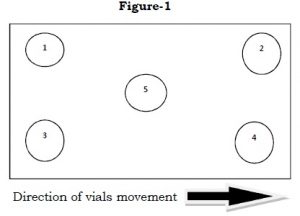
- Calculate the Average Velocity V of each HEPA filter as:
- Results:Verify the results and attach the report.
-
Air Flow Pattern Studies:
Objective:
To verify the unidirectional parallel airflow throughout the tunnel and the capability of the HEPA Filter unit of all zones to limit the dispersion and turbulence of air so as to maintain desired environmental conditions
Test Requirements:
Smoke Generator.
Protective aids.
Camera.
Test Procedure:
- Carry out the studies in unloaded conditions.
- The temperature of sterilizing zone of the Depyrogenating tunnel should not be more than 5°C above the ambient temperature during the test.
- Switch “ON” the blowers of the Depyrogenating Tunnel. Let the blowers run for 15 minutes for zones stabilization. Keep the heaters “OFF”.
- Hold the smoke generator pointing in the direction of airflow.
- Verify the path of smoke.
- Check the airflow at the tunnel outfeed and infeed points take photographs/ video graphs at each point.
Acceptance Criteria:
The Smoke Flow Pattern should coincide with the designed specifications laid down in the dehydrogenating tunnel manual and should move across the conveyor belt from the HEPA filter face in all the zones from drying zone to ambient.
Results:
Verify the results and attach the report.
-
Differential Pressure Monitoring:
Objective:
The objective of this test is to show that the differential pressures of followings Sterilizing and Depyrogenating Tunnel are within designed values:
- DP across Inlet chamber w.r.t. Environment
- DP across cooling chamber w.r.t. Environment
- DP across the HEPA filter of Inlet Chamber
- DP across the HEPA filter of sterilizing Chamber
- DP across the HEPA filter of Cooling Chamber
Test Requirements:
Calibrated Magnehelic Gauges and pressure transmitters fitted with Sterilizing and Depyrogenating Tunnel.
Test Procedure:
- Carry out the Differential Pressure measurement studies.
- Check the zero level of Magnehelic gauges.
- Operate the Sterilizing and Depyrogenating Tunnel as per
- Record the pressure differentials after the Sterilizing and Depyrogenation temperature in the heating zone is achieved.
- Pressure differential monitoring should be carried out three times at the interval of 10 Minutes during the operation in an empty chamber cycle and in the loading tunnel with every pack size.
Acceptance Criteria:
Differential pressures between areas of different zones of Sterilizing and Depyrogenating Tunnel with respect to Environment and differential pressure across the HEPA filters of different zones in the above conditions shall be as defined in the following figure & table.
| Parameters | Pressure Differential (Pascal ) |
| ∆P across the inlet chamber HEPA | |
| ∆P across the sterilizing chamber HEPA | |
| ∆P across the cooling chamber HEPA | |
| ∆P of drying zone w.r.t Environment | |
| ∆P of cooling zone w.r.t Environment |
Scientific rationale:
The initial pressure drop and final pressure drop ∆P across the HEPA should be as per filter certificates provided by the vendor, in this case, the filter manufacturer did not provide the IPD and FPD, So, The initial pressure drop ∆P across the HEPA is defined as per initial pressure identified during OQ and final pressure drop is defined as per vendor document.
Results:
Take the reading of differential pressure and the observation of the same shall be recorded in Test datasheet enclosed as Form No. 02.
- Non-Viable Particle monitoring:
Objective:
To establish the environmental conditions for Non-Viable Particulates inside the Sterilizing and Depyrogenating Tunnel.
Test Requirements:
- Calibrated Air Borne Particle Counter and thermal Paper rolls.
- Test Procedure:
- The test shall be carried for all three zones by taking 3 consecutive samples from each location for three consecutive days during initial qualification & for one day during re-qualification.
- Carry out the studies with Empty Tunnel.
- The temperature of sterilizing chamber of the Tunnel should not be more than 5°C above the ambient temperature during the test.
- Switch “ON” the Blowers of the tunnel. Keep the Heaters “OFF”. Let the blowers run for 15 minutes for zones stabilization. Place the receptor probe at about 6” from the surface and periphery of each HEPA Filter.
Acceptance Criteria:
The particle count measured should meet the criteria defined in the following table
| Condition | Particles/cubic feet | |
| ³ 0.5m | ³ 5.0 m | |
| Empty chamber for Three days during initial qualification & one day for Requalification | NMT 100 | Nil |
Results:
Verify the results and attach with the report.
-
Viable particle Count:
Objective:
To monitor the environment of the Sterilizing and Depyrogenating Tunnel with respect to viable particle count.
Test Requirements:
- Air Sampler.
- Pre-incubated Media Plates.
Test Method:
- Carry out the test at Blower “ON” and Heater “OFF” conditions.
- Two types of tests shall be carried out under microbial monitoring i.e. Active Sampling and Passive Sampling for a single day.
- Inlet and sterilizing chambers are monitored for information and Cooling chamber to establish Class 100.
Passive Air Sampling:
- Transfer the pre-incubated media plates and accessories to the area to be monitored in a closed container to avoid any contamination. Write the following details on the base of the plate (the part containing the media). Do not mark the lid of the plate.
- Place the plates on the sampling locations and remove the lid of the plate in such a way that the entire agar surface is completely exposed to the environment.
- Take care not to put fingers on plates and avoid passing anything over the top of the exposed media plate.
- Leave plates exposed for contact with the environment for 4 Hrs.
- After completion of exposed time close the plate by replacing the lid on the plate.
- Collect all exposed media plates in closed container to avoid any contamination and incubate them.
- Incubate the media plate (s) along with Negative control of the same media lot in inverted position in a incubator at 30 – 35°C for 5 days.
- Perform passive sampling and carry out the microbial analysis as per SOP.
Active Air Sampling:
- Transfer the incubated media plate and accessories to the area to be monitored in a closed container to avoid any contamination.
- Write the following details on the base of the plate the part containing the media. Do not mark the lid of the plate.
- Place the sampler on the sampling location to be monitored.
- Open the lid of the media plate/cassette and insert the sampling head of the air sampler and run the air sampler for the specified time for sampling 1000 lts. of air as per SOP.
- Take care not to put fingers on plates.
- After completion of sampling, cover the media plate with a lid.
- Incubate the media plate (s) along with Negative control of the same media lot in an inverted position in an incubator at 30 – 35°C for 5 days.
- Perform Active air sampling and carry out the microbial analysis is as per SOP.
- After completion of the qualification, statically calculations with charts shall be prepared and recorded in the report.
Acceptance Criteria:
| Microbial Limits | Limit for microbial monitoring | |
| Air Sample Cfu/m3 | Settle plate (Dia. 90mm) Cfu/ 4 Hour) | |
| Class 100 | <1 | <1 |
Observations and Results:
The report and the observation shall be recorded in the Test datasheet enclosed as Form No. 03. For Passive air analysis and Form No. 04 for active air analysis.
-
Empty Tunnel Heat Distribution Studies:
Objective:
To perform the temperature mapping inside the Sterilizing and Depyrogenating Tunnel in empty conditions and to ensure that temperature achievement in the whole sterilizing chamber should be above the minimum Depyrogenating temperature for the minimum required time.
Test Requirements:
Calibrated Data logger with calibrated Thermocouples (16 Nos.)
Test Procedure:
- Before starting qualification compare the time of Tunnel, Data logger with respect to the real time and record the observation.
- Place one probe in the center of Sterilizing zone (Probe should not touch conveyor belt and any metallic surface) shall be act as reference temperature probe of tunnel.
- The test shall be carried out as per test matrix.
- The temperature of the Sterilizing zone should reach the minimum set value before the loading of vials in tunnel.
-
Fix 15 pre-calibrated Thermocouples to the outside of an SS zig with heat resistant tape.
- Use Teflon tapes to secure probes in position. Ensure that the tips of probes where the wires are soldered do not touch any metallic surface.
- Connect the probes to a suitable data logger, which can scan and print the actual temperature observed at different locations with respect to time.
- Operate the Depyrogenating Tunnel as per procedure in SOP also start the data logger to record actual temperatures within the Depyrogenating Tunnel with respect to time at a scan time of 10 sec interval.
- Perform full loop calibration (probe + data logger) before starting the qualification runs. Attach calibration reports.
- Down load all the data from the data logger to the computer and do the temperature mapping.
- When the cycle completes; (1) Collect thermograph from the multipoint temperature recorder of the Depyrogenating Tunnel and (2) Download the data from data logger into the computer for data-analysis and printing. Record the temperatures observed at different locations.
- Perform full loop calibration (probe + data logger) before starting the qualification runs. Attach calibration reports.
- Down load all the data from the data logger to the computer and do the temperature mapping.
- When the cycle completes; (1) Collect thermograph from the multipoint temperature recorder of the Depyrogenating Tunnel and (2) Download the data from data logger into the computer for data-analysis and printing. Record the temperatures observed at different locations.
- Calculate value of each temperature mapping location.
- If the empty tunnel heat distribution study is acceptable perform next two consecutive replicate runs to demonstrate cycle and Depyrogenating Tunnel reproducibility (As per Test Matrix).
- Compile the data generated during the qualification test for complete evaluation of the system.
- After completion of qualification, statistical calculation with charts shall be prepared & recorded in the report.
- Prepare summary and conclusion of the performance qualification test, which will be finally approved by Quality Assurance.
Acceptance Criteria:
The Sterilizing zone of tunnel should meet the following criteria.
- The temperature attainment in all external temperature probes should not be less than 296.4 °
- The time of exposure in all temperature probes in the Sterilizing zone should not be less than 4 minutes.
- The FH Value should not be less than 30 minutes.
Rationale for temperature criteria:
- This is based on the exposure temperature of 250 °C for 30 minutes and Z value of 46.4° Increase in temperature from base temperature by 46.4°C will reduce the exposure time by 1 log i.e. 296.4 degree for 3 minute.
- The FH Value should be more than 30 minutes.
- Considering minimum requirement of 296.4°C exposure temperature and 3 minute exposure time, the FH value will be 30 minute as per the above formula.
- Considering the safer side for minimum exposure time, the acceptance criteria for minimum exposure time is given as 4 minutes.
- The predefined cycle parameter by vendor is decided based on the approach of comparative low temperature, longer exposure duration and minimum required lethality.
Result
Record the observations and attach the print out of the data logger in Test data sheet enclosed as Form No. 05.
-
Loaded Tunnel Heat Penetration Studies:
Objective:
- To perform the temperature mapping throughout the Depyrogenating Tunnel in the loaded conditions.
- To ensure the desired lethality inside the glass vials.
- To ensure the required log reduction inside the vials.
Test Requirements
- Calibrated Data logger with calibrated Thermocouple (21 Nos.)
- Endotoxin Indicator containing 10,000 EU/vial.
Test Procedure
- Before starting qualification compare the time of Tunnel, Data logger with respect to the real time and record the observation.
- Place one probe in the hot zone (Probe should not touch conveyor belt and any metallic surface) shall be act as reference temperature probe of tunnel.
- The test shall be carried out as per the matrix listed above.
- Carry out the Loaded tunnel Heat Penetration Studies after the Empty tunnel Heat Distribution Studies.
- The temperature of the Depyrogenating zone should reach the minimum set temperature for particular pack size before the subsequent loading of the vials.
************************************************END*****************************************



Pingback: Bacterial Endotoxin (LAL) Test Procedure - Pharma Beginners
Pingback: Dry Heat Sterilizer (DHS) Validation Protocol - Pharma Beginners