Standard Operating Procedure (SOP) & guideline for the Handling of Outliers or aberrant or Out of Specification test result in Near Infrared (NIR) Analysis.
Handling of Outliers in Near Infrared (NIR) Analysis
1.0 Purpose:
-
- The purpose of this SOP is to describe the procedure for the Handling of Outliers in Near Infrared (NIR) Analysis.
2.0 Scope:
-
- This SOP is applicable for the handling of Outlier or aberrant or Out of Specification test result in Near Infrared (NIR) analysis for Qualitative (Identification) purpose at the Quality Control Department.
3.0 Reference & Attachments :
-
- References:
-
- European Pharmacopoeia
-
- SOP for Handling of Out of Specification (OOS) Test Result
-
- Attachments:
-
- Attachment–1: Format for outlier handling of Near Infrared (NIR) Analysis Flow chart
-
- Attachment–2: Format for Near Infrared (NIR) Outlier handling form.
4.0 Responsibility – SOP for Near Infrared (NIR) Outlier :
-
- Analyst:
-
- To operate the Near Infrared (NIR) instrument as per SOP.
-
- To prepare and maintain the library of the standard with respect to the product, packing material, and its physical behavior.
-
- In case of outlier inform to the Head -QC or designee.
-
- Head Quality control or Designee:
-
- Training to be given to the analyst.
-
- To ensure library preparation, proper handling of Instrument, and documentation as per SOP.
-
- To investigate the outliers
-
- QA of QC :
-
- To issue the form of Investigation of Out of Specification Analysis Result
-
- Quality Assurance:
-
- To check the SOP.
-
- Ensure the implementation of the system as per SOP.
-
- To ensure the SOP is implemented.
-
- To review and approve the SOP.
5.0 Abbreviations– SOP for Near Infrared (NIR) Outlier:
-
- NIR: Near-Infrared Spectroscopy
-
- OOS: Out of Specification
-
- SOP: Standard Operating Procedure
-
- CAPA: Corrective Action and Preventive Action
-
- QC: Quality Control
-
- QA: Quality Assurance
-
- AR.No.: Analytical Report Number
-
- NA: Not Applicable
6.0 Procedure – SOP for Near Infrared (NIR) Outlier:
-
- In case any material identification is claimed as fail / rejected by Near Infrared (NIR) instrument, the analyst shall inform the Head QC or designee.
-
- The result shall be referred to as an outlier.
-
- The Head QC or designee shall inform to QA of QC for the issuance of format for Near Infrared (NIR) outlier handling form (attachment-2).
-
- QA of QC shall issue the number and keep the related record in the register as per OOS SOP.
-
- The issuance number shall be issued the same as for the OOS number allotment procedure.
-
- Head QC or designee shall carry out the investigation as per the checklist but not limited to, checklist provided in the attachment-2.
-
-
Discussion with the analyst for following but not limited to:
-
-
- Sample storage condition (if any) and its physical nature.
-
- Instrument operation and malfunction.
-
- Reference selection from the library.
-
- Packing material is the same as per the requirement and against the library.
-
- Analysis of the sample performed the same as the reference created.
-
- Enter the comment in the summary of the investigation based on previous experience, discussion with the analyst, and finding shall be reviewed and signed by head QC or designee.
-
- The investigation shall be carried out as follows.
-
-
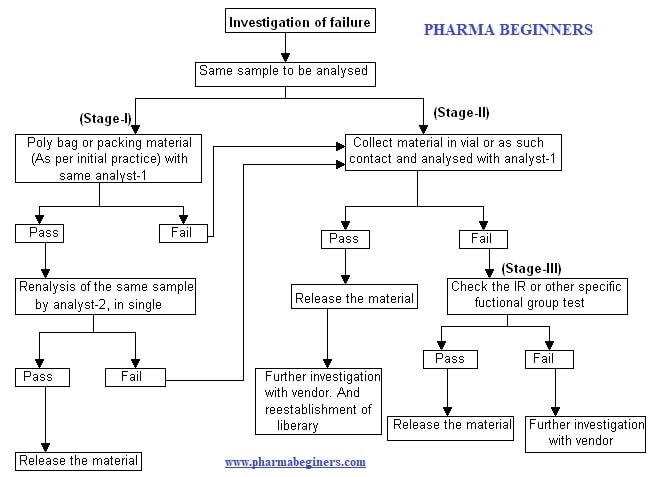
Stage-I: SOP for Near Infrared (NIR) Outlier:
-
-
- The same sample shall be taken using the same condition for the investigation in the polybag (packing material as per initially performed) with the same analyst-1, to confirm the analytical error or sample handling
-
- If the sample passes then reanalyze the sample with analyst -2 in single.
-
- When the sample passes then release the material
-
- If the sample failed in either with analyst-1 or with analyst-2 of the stage-I then the same sample shall be analyzed as per stage-II.
-
-
Stage-II: SOP for Near Infrared (NIR) Outlier:
-
-
- In the case of a non-established vendor, stage-II shall be directly followed for further investigation.
-
- The sample shall be analyzed by taking in the clear transparent vial or by as such contact (on separate butter paper) to the sample with the same analyst (analyst-1), to confirm the outlier due to the packing material.
-
- If the sample gets passed then release the material and further investigation with the vendor and re-established the library.
-
- In case, if the sample gets failed then the sample shall be analyzed as per stage-III.
-
-
Stage-III: SOP for Near Infrared (NIR) Outlier:
-
-
- Analyze the fresh sample by the IR method (if applicable) or other specific functional group methods to confirm the material identity.
-
- If the sample passes then release the material.
-
- In case if the sample gets failed then further sample failure to be investigated with the vendor.
-
- After investigation, QA of QC shall ensure the necessary attachment filed with the investigation report.
-
- After review of the investigation, a CAPA shall be issued by QA of QC and ensure compliance.
Attachment–1: Format for outlier handling of Near Infrared (NIR) Analysis Flow chart
Attachment–2: Format for Near Infrared (NIR) Outlier handling form.
| Form No. | : | Issued by | : | ||
| Issued to | : | Date | : | ||
| Material : | Manufacturer : | ||||
| Batch No. / AR No. : | Container No. : | ||||
| Results : | |||||
| Analyzed by : | Date : | Reference : | |||
Investigation – Handling of Outliers in Near Infrared (NIR):
| Sr.No. | Parameters | Observation | Sign / Date |
| 1 | Reference record/ document review | ||
| 1.1 | Checked for Reference library selected for the product is with correct requirement (ie, water content, polymorphic form, particle size etc.) | ||
| 1.2 | Checked for method of analysis followed, as per NIR SOP. | ||
| 2 | Sample check | ||
| 2.1 | Check the sample for physical examination for any abnormalities. | ||
| 2.2 | Check sample stored as per the storage condition,if any. | ||
| 2.3 | Check for the packing material is same as per the requirement and against the library. | ||
| 3 | Instruments review | ||
| 3.1 | Instrument PQ status to be checked. Name of Instrument :_____________ Code No.: _________________ |
| 3.2 | Check instrument parameters where applied similar to library creation (Like : threshold, no. of scans, background correction etc.) | ||
| 4 | Sample preparation investigation: | ||
| 4.2 | Analysis of sample performed in same manner as performed during the creation of reference library. | ||
| 4.3 | Sample age to be taken in consideration at the time of library creation. | ||
| 4.4 | Sample is evaluated against the specified vendor reference only (If require). | ||
| 5 | Analyst Check: | ||
| 5.1 | Check analyst is trained for the analytical technique to perform particular analysis. | ||
| 6 | Other review: | ||
| 6.1 | Evaluate the environmental temperature/ humidity condition, where the test was performed. |
Summary of discussion points
| Sr. No. | Summary of discussion points | Remark of Investigator |
| 1.
2. 3. 4. |
Under standing of analytical technique:
Sample handling during analysis: Any abnormality observed during sampling and analysis: Any Other |
|
| Summary of the investigation by the investigator (Previous experience / Based on analysis / Discussion) : | ||
| Section head: QA of QC:
Date : Date : |
||
*************************************************END**********************************************


