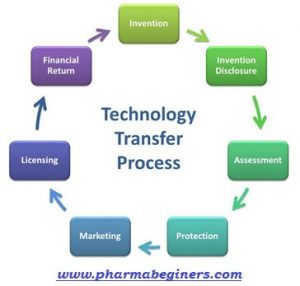
 Technology transfer/product transfer is a complete documented process that covers the detailed documentation for manufacturing and dispatch of the first batch, received from the parent location or FDD of a new or existing product from a particular site with manufacturing, packaging, and analytical details.
Technology transfer/product transfer is a complete documented process that covers the detailed documentation for manufacturing and dispatch of the first batch, received from the parent location or FDD of a new or existing product from a particular site with manufacturing, packaging, and analytical details.
SOP on Technology Transfer of Drug Product
1.0 PURPOSE:
-
- The purpose of this SOP is to define the procedure to be followed for a successful transfer of new technology or product (Technology Transfer of Drug Product) from-
-
-
- One manufacturing site to another site of same manufacturing group/company or
-
-
-
- Formulation Development Department (FDD) / R&D to manufacturing site.
-
2.0 SCOPE :
-
- Scope of this SOP covers
-
-
- All existing products being transferred from one site to another site and
-
-
-
- New products from FDD.
-
3.0 REFERENCES:
-
- SOP for Risk Management
-
- The analytical Method Transfer procedure
-
- Change Control Procedure
4.0 RESPONSIBILITY:
-
-
Formulation Development (FDD):-
- Responsible to provide Technology transfer documents (MPS/PIF) to location.
-
-
-
- Organize a meeting with location after circulation of above documents,
-
-
-
- if required, Present at location while manufacturing of initial batches.
-
Analytical Development Department (ADD):-
- Responsible to provide required documents like,
-
-
-
-
- Specification,
-
-
-
-
-
- Analytical Test Procedure,
-
-
-
-
-
- Analytical Method Validation and
-
-
-
-
-
- Organize a meeting with location after circulation of above documents, and
-
-
-
-
- if required, Guide /support and demonstrate the skill involved in the analysis as per requirement.
-
Packaging Development Department (PDD):-
- Responsible to provide required documents like-
-
-
-
-
- Approved artworks,
-
-
-
-
-
- Packaging material descriptions
-
-
-
-
-
- Pack profile to the location
-
-
-
-
-
- Participate in new product launch meetings and process development/scale-up trials if required.
-
-
Production Officer / Executive:-
- Responsible for review of-
-
-
-
-
- Product Information File (PIF) / Master Product Specification (MPS) on receipt,
-
-
-
-
-
- Plan for batch,
-
-
-
-
-
- Raise Purchase Request (PR) for additional facility required,
-
-
-
-
-
- Up-load the Bill of Material (BOM) in Metis or other adopted software and
-
-
-
-
-
- Prepare of Batch Manufacturing Records (BMR) / Batch Packing Record (BPR), Risk Assessment Report (FMEA).
-
-
Warehouse Dept.:-
- Responsible to Raise Purchase Requests for Raw materials / Packing materials, check availability of materials.
-
-
-
- Ensure that materials are receipt & stored as per the SOP of receipt.
-
-
-
- Storage of materials.
-
-
-
- Dispensing of material as per the batch manufacturing plan.
-
QA Officer / Executive:-
- Responsible for receiving, reviewing, and control of technology/product transfer-related documents.
-
-
-
- Review of the PIF/MPS on receipt, authorize, issue the BMR / BPR, plan validation activity as per validation plan,
-
-
-
- Review Risk Assessment Report / FMEA report and evaluate mitigation plan prior to the manufacturing of batches.
-
QC Officer / executive:-
- Responsible for review and preparation of specifications,
-
-
-
- Preparation of Standard Testing Procedure (STP), Analytical Test Data Sheet and
-
-
-
- Qualification of working standards in accordance with the product requirements.
-
Bonded Store Room (BSR) officer/ executive:-
- Responsible for proper storage and dispatch of the finished product after release.
-
5.0 DEFINITION:
-
-
Technology Transfer:
- Technology transfer/product transfer is a complete documented process that covers the detailed documentation for manufacturing and dispatch of the first batch, received from the parent location or FDD of a new or existing product from a particular site with manufacturing, packaging, and analytical details.
-
Master Product Specification (MPS):
- The MPS most importantly have below information but not limited to the following,
-
-
-
- ERP code for, Active pharmaceuticals ingredient (API), Excipients, and Primary packing material/s.
-
-
-
- Drug substance, excipients & primary packaging material sourcing.
-
-
-
- Detailed Manufacturing formula along with the bill of materials (BOM).
-
-
-
- Precautions / Handling / Storage / Sampling details where ever required.
-
-
-
- Brief manufacturing process.
-
-
-
- Critical equipment list/critical process requirements.
-
-
-
- Required utility/consumable list.
-
-
-
- Information regarding tooling/change parts (size, shape, diagram /drawing).
-
-
-
- Primary packing material specifications.
-
-
-
- In-process specification and finished product specification.
-
-
-
- Stability data and MSDS (API / Excipient / Finished product), Draft Label, and Flow chart.
-
6.0 PROCEDURE FOR TECHNOLOGY TRANSFER:
-
-
Technology Transfer of New Product/ Transferred Product (Domestic):
- FDD/ADD or Transferring location/Site transfer all the documents related to technology transfer of new batch/ transferred batch to the site.
-
-
-
- Intimate the site QA for any additional document required and ensure the availability of the same.
-
-
-
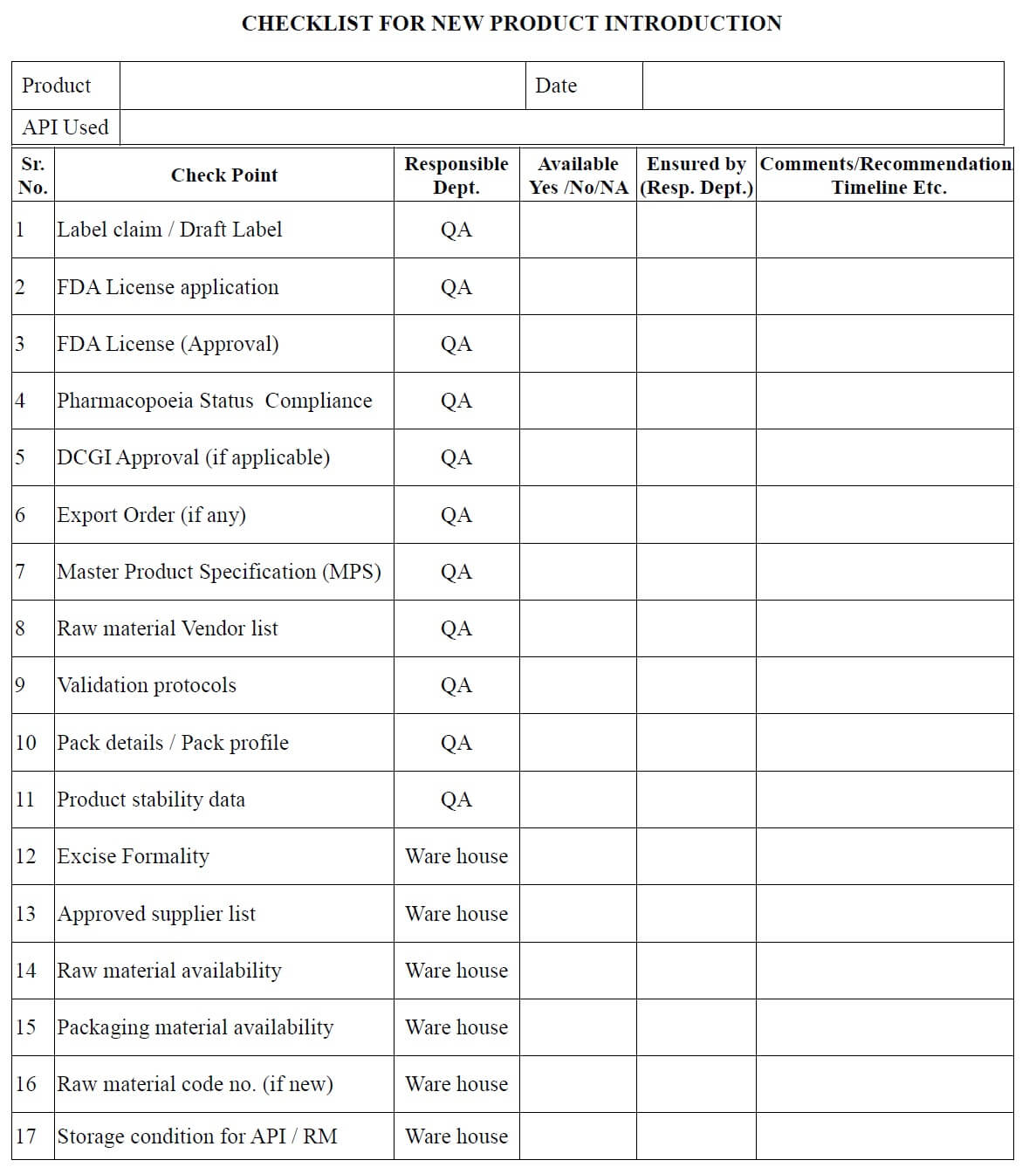
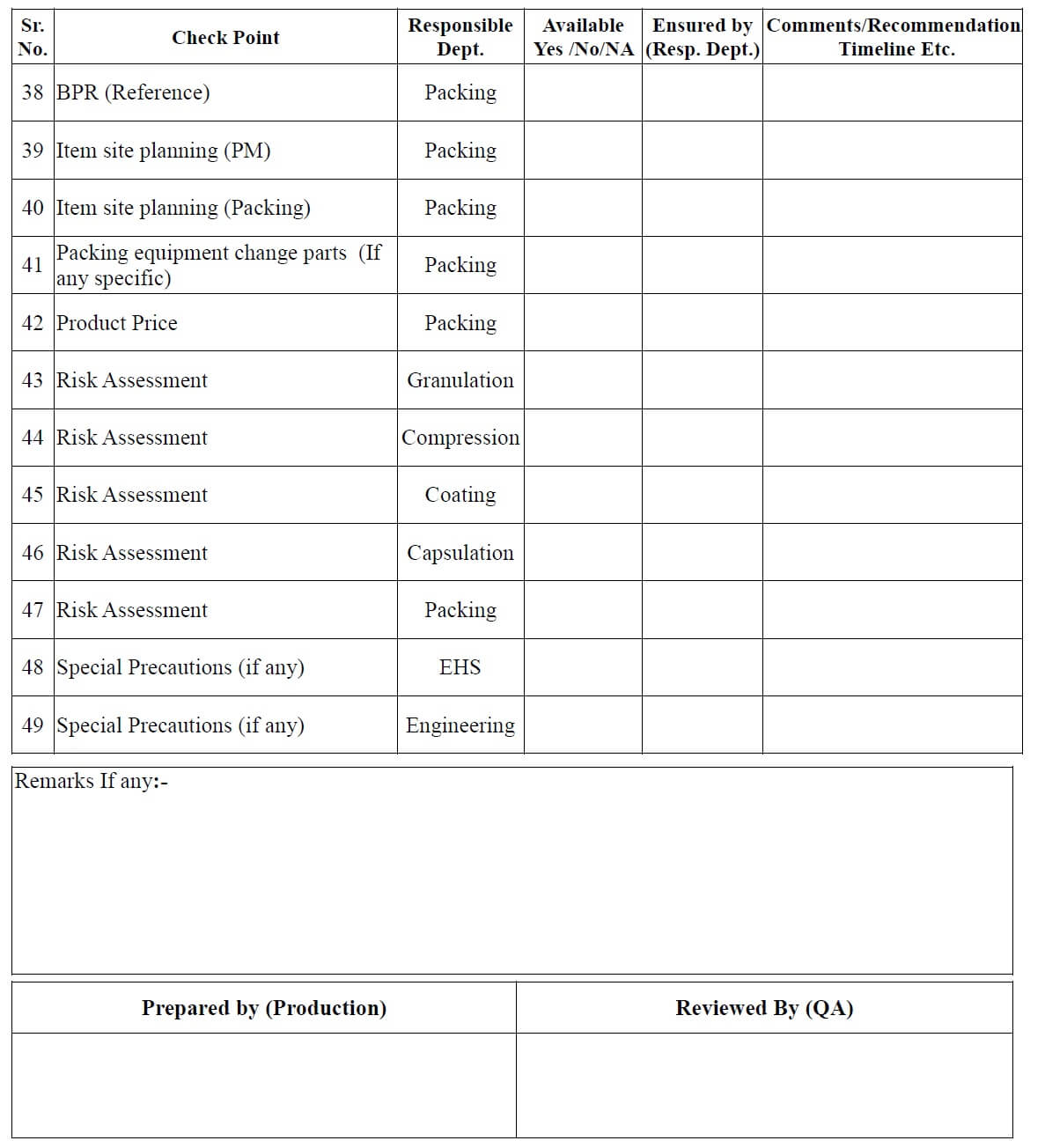
- After receiving the documents from the FDD/ADD or transferring location/site, QA officer make the necessary entries in the checklist for new / transferred product (Annexure 1) and
-
-
-
- Issue it to all involved departments for checking of documents related to their concerned area.
-
-
-
- All involved departments check the technology transfer document and fill the checklist (Annexure-1) and returned it to the QA department.
-
-
-
- QA Executive/officer can also fill the checklist for new product transfer in consultation with the concerned department heads.
-
-
-
- QA Executive/officer reviews the readiness/availability of documents.
-
Change control Initiation:
- Production personnel can initiate the change control procedure for the manufacturing process.
-
Technology Transfer of Analytical Method:
- Handle the technology transfer of analytical method as per SOP of Analytical Method Transfer.
-
-
-
- Intimate for required precaution/measures in analytical method to site QC from ADD.
-
Procurement Procedure:
- After completion of checking provision, all concerned departments initiate the procurement of raw materials/packing materials/change parts and planning for manufacturing of new products/site transferred product.
-
Procurement of Raw Materials and Packaging Materials:
- Procurement of raw material and packaging material initiated as per batch size decided in the planning from the source/vendor mentioned in MPS.
-
-
-
- Warehouse raise the purchase order for the raw material and packaging materials.
-
Procurement of Tooling / Change Parts:
- The production department raise the purchase order for the tooling as per the drawings supplied by FDD along with MPS, based on
-
-
-
-
- Tablets size,
-
-
-
-
-
- Shape,
-
-
-
-
-
- Quantity and
-
-
-
-
-
- Type of tooling (B or D) for available tablet press.
-
-
-
-
- Mention special precautions/features required for tooling in MPS if specified by FDD.
-
-
-
- For example, lower punch with key and hard chrome plating on punches.
-
-
-
- Production department / PDD orders the capsules to change parts as per the size and requirement by FDD along with MPS.
-
-
-
- Packing department / PDD orders the packing change parts as per the size and requirement by FDD along with MPS.
-
-
-
-
Once all raw materials, packing materials, change parts, tooling, and FDA permission for the product are received, FDD be informed for support to establish the manufacturing parameters.
-
-
-
-
- Manufacturing BOM, MBMR, packing BOM, MBPR, Spec./ ATP of all respective products to be prepared prior to the manufacturing plant.
-
-
-
- Make sure the availability of Raw material and packing material prior to the manufacturing plant.
-
-
-
- Prior to the manufacturing of the first batch (Issuance of BMR for the first batch) of the product on-site,
-
-
-
- If required, the first batch of new / transferred product manufactured in presence of FDD / transferring site Production personnel,
-
-
-
- Therefore to know about the critical or key step as well as a precaution needed to be taken while manufacturing commercial batches.
-
-
-
- Based upon the first three batches, Hence in case of any changes to be incorporated, FDD revises the MPS and accordingly the MBMR / MBPR prepared.
-
-
-
- The stability sample is withdrawn by IPQA & performs stability study as per SOP of the “Stability Program”.
-
-
-
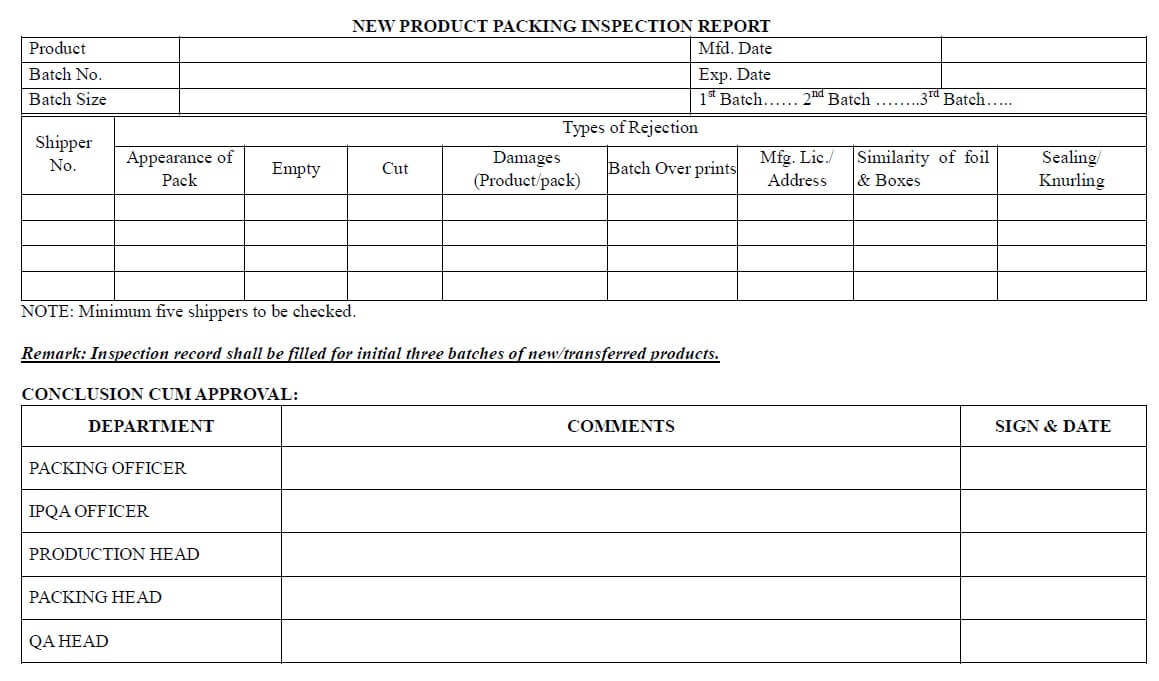
- For the first three batches, an “Inspection report” most importantly filled by the packing officer and cross-verified by the IPQA officer.
-
-
-
- Minimum 5 packed shippers checked for the first three batches during its packaging.
-
-
-
- After completion of packing process (during the closure of BPR), Finally the batch release authorization for New Product filled by QA prior to the release of batches (For 1st three commercial batches).
-
6.0 ABBREVIATIONS:
-
- ADD : Analytical Development Department
-
- API : Active Pharmaceutical Ingredient
-
- BMR : Batch Manufacturing Record
-
- BPR : Batch Packing Record
-
- BOM : Bill of material
-
- COA : Certificate of Analysis
-
- CQ : Corporate Quality
-
- ERP : Enterprise resources planning
-
- FDA : Food and Drug Administration
-
- FDD : Formulation Development Department
-
- FMEA : Failure Mode Effect Analysis
-
- LIMS : Laboratory Information Management System
-
- MF : Master Formula
-
- MFC : Master Formula Card
-
- MPS : Master Product Specification
-
- MSDS : Material Safety Data Sheet
-
- PDD : Packaging Development Department
-
- PIF : Project Information Form
-
- PR : Purchase Requisition
-
- QA : Quality Assurance
-
- QC : Quality Control
-
- R & D : Research and Development
-
- RS : Reference Standard
-
- SOP : Standard Operating Procedure
-
- STP : Standard Test Procedure
-
- WRS : Working Reference Standard
7.0 ANNEXURES
Annexure – 1
Annexure – 2
New Product Manufacturing Authorization
| Product | Dosage form | ||
| API Used | |||
FOR COMMERCIAL PRODUCT
| Sr. No. | Requirements | Available
Yes/ No |
Ensured By
(QA) |
Comments/ Commitments |
| 1 | D.C.G.I. permission (If Applicable) | |||
| 2 | F.D.A. Manufacturing License | |||
| 3 | Excise formalities (if any). | |||
| 4 | Raw material specification | |||
| 5 | Packing material specifications | |||
| 6 | Finish Product specification | |||
| 7 | Batch manufacturing record | |||
| 8 | Batch Packing record | |||
| 9 | Raw materials availability | |||
| 10 | Packing materials availability | |||
| 11 | Data on material safety, excipients handling or any critical controls for environment, storage and product (If any) | |||
| 12 | Availability of accessories and machinery parts for manufacturing as well as packaging (punches, spares, etc.) |
|
||
| 13 | Analytical method | |||
| 14 | Reagents, reference standards, working standards and impurity standards | |||
| 15 | Process Validation Protocol, sampling tools. | |||
| 16 | Any other special requirement (……………………………………………) | |||
| 17 | Any other special requirement (……………………………………………) |
Based on the available data, we hereby authorize the product to be released for manufacturing subject to above criteria being satisfied.
| Department | Comments | Sign & Date |
| Warehouse Head | ||
| Manufacturing Head | ||
| Packing Head | ||
| QC Head | ||
| Engineering Head | ||
| EHS Head | ||
| Regulatory Affairs | ||
| Production Head | ||
| QA Head | ||
| Head Quality | ||
| Plant Head |
Annexure – 3
New Product Packing Inspection Report
Also visit : Raman Analyzer SOP – Click



Pingback: New Product Introduction (Risk Evaluation) - Pharma Beginners
Pingback: Vendor Management - SOP and Complete Guide - Pharma Beginners
Pingback: Stability Study SOP as per ICH Guideline - Pharma Beginners
Pingback: Process Validation : New Approach (SOP / Protocol) - Pharma Beginners