Annual Product Review (APR) is the requirement of various regulatory agencies. APR roots the monitoring of product quality as well as finding out the scope of quality improvement by controlling the critical process parameters (CPP).
APR – Annual Product Review also known as APQR – Annual Product Quality Review and PQR – Product Quality Review, below the SOP and formats for APR preparation.
Annual Product Report (APR/APQR/PQR):
Annual product report is a documented evidence for assuring that the various manufacturing parameters are controlled enough which results into a finished product meeting all predetermined specification and other quality attributes. Also acts as an indicator to identify requirement of changes in specifications or manufacturing processes or control parameters with the help of statistical review of trend.
SOP for Annual Product Quality Review (APR / APQR / PQR)
Purpose:
The purpose of this sop is to describe the detail procedure for preparation, review and approval of annual product report/ product quality review (APQR / APR /PQR) with the objective of verifying the consistency of the process, equipment and system for meeting predetermined specifications and other quality attributes of a finished product.
Scope:
This procedure applies to all drug products manufactured to understand and review the process, Specification and adherence to specified standards.
Note: APR / APQR / PQR, all are same but the terms are differently used in guidelines.
References & Annexures:
-
References
- Guideline for preparation of annual Product review of Drug Products.
- 21 CFR Part 211.
- Good Manufacturing Practices issued By WHO.
- Australian code of cGMP for medical products issued by TGA.
-
Annexures:
- Format for Annual Product Review Tracking Register
Responsibilities for preparation of Annual Product Review – APR:
-
Quality Assurance:
- Compile and review the data from relevant departments and prepare Annual Product Review (APR) report as per defined procedure.
- Prepare and review graphical representation.
- Prepare qualification status of relevant equipment’s & utilities and any other relevant details respect to Annual Product Review.
-
Quality Control:
- Provide current specification of API and Finished Product and any other relevant details with respect to Annual Product Review.
- Summarize results of stability monitoring program.
- To prepare data of QC events, investigations, OOS and provide their relevant investigation and effectiveness of relevant corrective and preventive actions taken.
- To provide current specifications of packing material.
-
QA Head or Designee:
- Review the Annual Product Review Report.
- Responsible for assuring that all the requirements of APR are fulfilled.
- Ensure the compliance of APR and submit the APR to corporate regulatory affairs for any submission.
Abbreviations and Definition of Terms used in SOP for Annual Product Review – APR:
-
Abbreviations :
- CC No.: Change Control number
- NA: Not Applicable
- QA: Quality Assurance
- QC: Quality Control
- APQR: Annual Product Quality Review.
- APR : Annual Product Review.
- PQR: Product Quality Review.
- PPk : process Performance index
- CPP : Critical Process Parameter
-
Definition of Terms :
- Trend: Trend is the tendency of data to exhibit an increasing / decreasing / cyclic pattern when the data is presented in a graphical manner. A change in a trend is usually associated with some cause.
Procedure – Annual Product Review (APR):
- Preparation of Annual Product Quality Review (APQR):
- Annual product report shall prepare for all finished products manufactured.
- APR to verify the consistency of the existing process, the appropriateness of current specifications for
- Raw materials.
- Packing materials.
- Intermediate product and
- Finished product to identify any emerging trends as also to identify product / process related improvements.
- APR shall prepare for drug Products as per below time line and acceptance criteria:
-
Time Line to prepare the Annual Product Quality Review :
Yearly+ 3 months.
-
- APR shall prepare for all products manufactured in a year.
- If more than fifteen batches manufactured during the review period, Prepare graphical presentation of analytical trend data of in-process and finished product.
- In case of less than fifteen batches, instead of graphical presentation, minimum/maximum value of trend shall be prepared and reported.
- APR tracking register shall be maintained.
-
Preparation of APR consists of three stages:
- Collection of data / information,
- Review of data / information and
- Generation of review report.
- Annual Product Reports shall contain data of all the batches manufactured for a product during calendar year from January to December.
- Separate APR / APQR shall generate in case a product is produced using different manufacturing process.
- Review shall cover all the markets where the product is being sold.
- In case of a product that is manufactured in multiple strengths or different packs, combined APR report can generate. however each pack or strength shall evaluate in separate manner.
- Preparation of APQR / APR / PQR of all products shall complete till the end of first quarter of product anniversary.
- To manage the preparation of APR of all products and to divide the work load throughout the year,
- APR of different products shall plan in different months i.e. as per product anniversary.
- Suppose 120 products are manufactured in year, then every month approx. ten products shall identifying for preparation of APR.
- The batches to be considered for APR review shall dependent on the APR period i.e. 01/01/14 to 31/12/14 or 01/02/14 to 31/01/15 or 01/03/14 to 29/02/15 so on.
- APR of different products shall plan in different months i.e. as per product anniversary.
- The next year review shall perform for next review period i.e. if in first year the period was selected the 01/01/14 to 31/12/14
- Then the next year APR shall prepare for the batches manufactured during the period from 01/01/15 to 31/12/15, for the products APR falling during this stated period and same shall follow for the rest.
- In-case of WIP for a product, APR shall complete after its final QA release.
-
General Instruction for preparation of Annual Product Review – APR :
- Various data incorporated into the APR can present in tabulated form. Also graphs, flow chat, etc. can use as per the requirement.
- APR of all products shall kept with QA department.
- All relevant points shall discuss with the concern department/s.
- APR shall not destroy in case of product transfer, product discontinuation or banned from govt. Authority.
- If the APR is required to share with other locations,
- Then the “Uncontrolled copy” shall issue upon request.
- If the APR is not due,
- Than only raw trend data shall share with the new location.
- In-case a particular product is not manufactured during a particular year,
- Then the APR shall restrict to review of only relevant points.
- Annual product report shall prepare in accordance to the following points.
- Each APR shall have a covering page which includes
- The Company Logo in the center of the page.
- “ANNUAL PRODUCT REPORT” below the Logo.
- The table shall contain the product name, Year and APR number.
- Subsequent pages of the APR shall bear the Header and footer.
- Header shall bear the Logo at the top right corner of each page.
- “ANNUAL PRODUCT REPORT “shall be written at top center of each page.
- Header shall contain the table at top of each page which shall contain the page no., Product name, Generic name and market.
- Footer of each page shall contain the unique APR number on left corner of each page.
-
The content of APR shall contains
- Objective
- Introduction
- Product details
- Process flow
- Review of manufacturing related Information:
- Batch
- Yield at various manufacturing stages:
- Rejections:
- Reprocessing / reworking:
- Review of critical quality parameters
- In-process and intermediate testing results:
- Review of Finished product testing results:
- Out of Specification:
- Review of
- Events:
- Changes:
- Market complaints:
- Control Samples:
- Recall and Returns:
- Stability Data:
- Validation and qualification status:
- Post marketing commitments:
- Quality of APIs, Key raw materials, printed packing materials,
- Primary packaging material:
- Technical agreement:
- Statistical evaluation of Data:
- Review of status against previous APR:
- Conclusion
- Recommendation
- Abbreviations
- Annexures
- Approval
- Objective shall describe the purpose of preparation of APR.
- Introduction shall describe the details of batches manufactured during the year.
-
Product details shall describe
- Name of the Product,
- Product code,
- Generic Name,
- Strength,
- Therapeutic activity,
- Batch size,
- Shelf life,
- Dosage form,
- Total number of batches manufactured,
- Manufacturing License. No,
- Storage condition,
- Manufacturing and expiry date.
- Other items can be incorporated to product details, if required.
- Process flow shall be presented through a flow chart diagram covering all critical manufacturing steps.
-
Review of manufacturing related Information – Annual Product Review – APR:
- Listed below are the manufacturing related parameters that will be reviewed as a part of APR, this shall cover all the stages involved in the manufacturing process (e.g. in case of tablet manufacturing process, stages involve are generally granulation, compression, coating and packing)
-
Critical process parameters:
- Tabulate the data generated for critical parameters for all the batches.
- Convert this data into graphical form and review the same for emerging trends / atypical pattern in the graphs.
- Determine whether any atypical pattern has impacted the quality of the final product.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Batch yield at various manufacturing stages:
- Tabulate the yield at various stages for all batches.
- Generate graphs and review the same for any emerging trends.
- Check whether investigation has been documented in case of batches not meeting the yield limit and check whether the root cause has been identified and whether corrective / preventive action/s taken were adequate.
- Check if there were repeated yield related events/s and evaluate whether the root cause identification and corrective / preventive actions were adequate or any additional actions need to be undertaken.
- Provide a brief summary in the APR report based on findings of the above points.
-
Batch Rejections:
- Check
- If any batches were rejected during the review period.
- Whether investigation for the batch has been documented.
- Adequacy of root cause identification and corrective / preventive actions, if any.
- Disposition status of the rejection batch.
- Provide a brief summary in the APR report based on findings of the above points.
- Check
-
Batch reprocessing / reworking:
- Check
- If any batches were reprocessed or reworked during the review period.
- The adequacy of root cause identification and corrective / preventive actions, if any.
- Whether the reprocessed /reworked batch met the specification and was charged for stability study.
- The disposition status of the reprocessed / rework batch.
- The impact on quality of reprocessed /rework batch.
- Provide a brief summary in the APR report based on findings of the above points.
- Check
-
Review of critical quality parameters:
- Listed below are the critical quality parameters that will be reviewed as a part of APR.
-
Review of Finished product testing results:
- Tabulate the analytical results for key quantitative tests conducted on the finished product batches in the APR report
- Convert this data into graphical form and check for emerging trends / atypical pattern in the graphs
- Determine, a change in the trend has any underlying cause.
- Compare the trend versus corresponding trends obtained for in process and intermediate samples to check. There is, any correlation or a cause effect scenario.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Out of Specification Results:
- Check the log for OOS noted during the review period for the product in question.
- Determine that the all OOS properly investigated and identify the root cause.
- Verify the corrective preventive actions documented in the investigation reports.
- Categorize the OOS results based on root cause to identify any recurrent pattern.
- Check the actions taken for recurrent type of OOS results are effective.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Review of Events:
- First of all, Check the log for events noted during the review period for the product in question.
- Then Verify, all the events has investigated and root cause identified and documented in the investigation report.
- Then Verify, the Corrective / preventive actions documented in the investigation reports have been actually been completed and the event report has been closed within stipulated time frame.
- Check there, Any recurrent events and whether actions taken for such events are effective.
- In-case of pending actions, check whether the delay is justified.
- then Categorize the events based on root cause to identify any recurrent pattern.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Review of Changes:
- Review the changes implemented during the period (changes to manufacturing process, analytical specifications and test methods, utility process equipment should be covered as a minimum)
- Check all the changes for closeness.
- Verify the post–implementation review of the change. It should adequately documented.
- Assess, there is a cumulative impact of all the changes on the product quality
- Then check for re-validation (if required).
- Provide a brief summary in the APR report based on the findings of the above points.
-
Review of market complaints:
- Check the log for market complaints and note the number of complaints received for the product in question.
- Check each complaint. For completeness of Investigation / root cause finding.
- Verify for corrective / preventive actions. It should documented in the investigation reports.
- In-case of pending actions, check whether the delay is justified.
- Categorize the complaints based on the root cause to identify any recurrent pattern.
- Check the actions taken for recurrent type of complaints.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Review of control Samples:
- A summary of periodic review of control samples (Physical observation) carried out during the review period shall be included in the APR report. Any visually abnormal findings noted for the control sample during such periodic review shall also be included in the APR.
-
Review of Recall and Returns:
- Note the number of batches recalled or returned.
- Check for stock reconciliation. Reported correctly.
- Check For each recall. For completeness of investigation/root cause identification.
- Verify the corrective / preventive actions documented in the investigation reports.
- Verify the disposition status of the stock.
- Categorize the recalls based on root cause to identify any recurrent pattern.
- Check there has any recurrent recall or returns/ recalls and
- Whether actions taken for such returns/ recalls are effective.
- In-case of pending actions, check whether the delay is justified.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Review of Stability Data:
- Check those batches that was taken up for stability study during the review period and the reason for the same.
- Review the stability data generated during the review period and note whether there have been any atypical / OOS/ stability failures / adverse trends.
- Review the outcome of the completed stability studies and recommendation if any.
- Verify whether actions has taken for stability failures.
- Provide whether actions has taken for stability failures.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Review of Validation and qualification status:
- Verify the validation / qualification status of the manufacturing process, relevant processing equipment critical utilities such as water system, HVAC system, compressed air, etc. Cleaning methodology, relevant analytical instruments and analytical methods.
- For outcome of the validation exercise.
- Review the validation documents(s) i.e. for the completeness of validation.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Review of Post marketing commitments:
- Include a summary of variations to marketing authorization that were submitted /granted/rejected.
- Review-
- The post marketing commitments for any changes made to the registered requirements and provide a brief summary about the same in the APR report.
- Quality of APIs, Key raw materials, printed packing materials, Primary packaging material:
- API Test Results: This activity will carry out by QC and report submitted to QA for review.
- The test result will review vendor wise to determine any change which may have a direct or indirect effect on the finished product.
- Based upon the trend result the vendor status may change from its present status.
- Check
- There has been any change in specification, test methods vendor for the above materials used in the product in question.
- The status of vendor qualification and identify vendors for which quality-audit is pending.
- Whether there were any rejections of above materials during the review period and the name of the vendor.
- For recurrent rejection of particular material from same vendor, check whether the supplier has submitted any investigation report and whether vendor’s actions were verified where required.
- In case of drug products, trending of key analytical results of API batches shall be carried out.
- Provide a brief summary in the APR report based on the findings of the above points.
-
Review of Technical agreement:
- Verification of the requirement for technical Agreement with customers
- It compiles and whether there have been changes to the agreement.
- Provide a brief summary in the APR report based on the findings of the above points.
- Verification of the requirement for technical Agreement with customers
-
Statistical evaluation of Data:
- Data for batch yield, in-process testing, intermediate testing, finished product testing should statistically evaluated to determine the maximum, minimum, Mean, standard Deviation and Relative standard deviation (RSD) values.
- The necessary changes or freez of limits ,wherever applicable as per the recommendation by using the formula given below.
Upper control limit: (Arithmetic mean + 3 X standard deviation (σ)).
Lower controlled limit: (Arithmetic mean – 3 X standard deviation (σ)).
In case the UCL or LCL falls out of specification limit,
-
- the minimum and/or maximum limit among all data for a particular parameter can consider as
- limit for recommendation or
- the root cause of such variation to identify to eliminate the same for future batches.
- the minimum and/or maximum limit among all data for a particular parameter can consider as
Process performance and Process performance index can calculate as a part of further study of trend results.
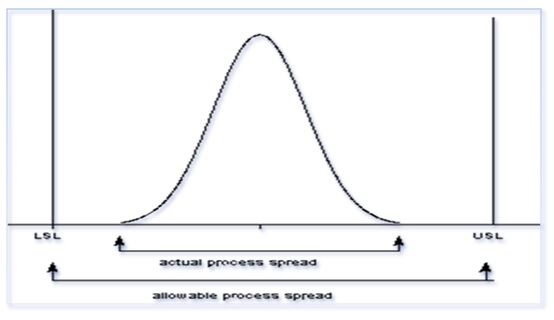
The process performance, or Pp, measures a process’s performance, which is defined as the allowable spread over the actual spread. (Refer Diagram below)
Process performance: Pp= USL-LSL/6σ
Where Pp= process Performance, USL= Upper Specification Limit,
LSL= Lower Specification Limit and σ= Standard deviation
-
-
Interpretation of Pp Value:
- As Pp is inversely proportional to the standard deviation, higher the value of Pp, better is the process performance.
- Process Performance index (Ppk) value shall calculate for batch yield, and to key quantitative results (Assay, Related substances, residual solvents etc.).
- Below the formula for calculation of Two-sided specification.
-
Formula- 1: Ppk=Pp-(m-x)/3S
Where: Ppk = process Performance index
m=Mean Point (USL+LSL/2)
x= Mean value
S: Standard Deviation
Formula -2: Ppk= Minimum of (USL- X) / 3S or (X- LSL) / 3S
Where: USL: Upper Specification Limit
LSL: Lower Specification Limit
X: Mean Data
S: Standard Deviation
-
-
Interpretation of PpK Value:
- If PpK ≥ 1
- Then the process is capable of generating 99.7 % of the product batches that are within the specification. If required, do the Further assessment.
- If PpK ≤ 1
- Then the process may generate some non-conforming batches over a period of time and needs assessment to identify and eliminate cause for variability.
- If required, do the Further assessment in this case.
- If PpK ≥ 1.33 :
- Then the process is capable, For normally distribution of population.
-
For more details about Process Capability calculation (cpk ppk value calculation) Click here
-
-
Review of status against previous APR:
- Check for any recommendations / actions stated in previous APR completed for the product.
- Compare the trends against those included in the previous APR for any similarities / differences, check whether any corrective actions completed in previous year have improved the trends during this year.
- Provide a brief summary in the APR report based on the above points.
-
Conclusion:
- A suitable conclusion shall drawn by reviewing the above mentioned parameters.
-
Recommendation:
- Based on logic and statistical review, recommendation for betterment of the product and system shall describe.
- Tools shall design to ensure the implementation of recommended action plan/s for betterment of future batches.
-
Addendum Report:
- Annual product report can reopen for incorporation of further information under circumstance’s as listed below. An addendum report shall prepare for further updation.
- If any information found missing while review.
- To include the recommendation/suggestion of auditors.
-
Annual Product Review Tracking Register
Also visit for : Function of QA in Pharmaceuticals <Click here)



Pingback: What is annual product review? - What Type Degree
Pingback: Change Control Management Procedure (SOP) - Pharma Beginners
Pingback: SOP - Corrective Action and Preventive Action (CAPA) - Pharma Beginners
Pingback: SOP for Quality Risk Management (Guideline ICH Q9) - Pharma Beginners
Pingback: GxP Record Retention and Archival Policy in Pharma - Pharma Beginners